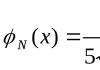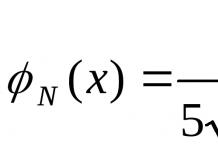One of the main characteristics of any chemical element is its relative atomic mass.
(An atomic mass unit is 1/12 of the mass of a carbon atom, the mass of which is taken to be 12 amu and is1,66 10 24 G.
By comparing the masses of atoms of elements with one amu, find the numerical values of the relative atomic mass(Ag).
The relative atomic mass of an element shows how many times the mass of its atom is greater than 1/12 the mass of a carbon atom.
For example, for oxygen Ar (O) = 15.9994, and for hydrogen Ar (H) = 1.0079.
For molecules of simple and complex substances, determine relative molecular weight, which is numerically equal to the sum of the atomic masses of all atoms that make up the molecule. For example, the molecular weight of water is H2O
Mg (H2O) = 2 1.0079 + 1 15.9994 = 18.0153.
Avogadro's law
In chemistry, along with units of mass and volume, a unit of quantity of a substance is used, called the mole.
!MOL (v) - a unit of measurement of the amount of a substance containing as many structural units (molecules, atoms, ions) as there are atoms contained in 0.012 kg (12 g) of the carbon isotope “C’’.
This means that 1 mole of any substance contains the same number of structural units, equal to 6,02 10 23 . This quantity is called Avogadro's constant(designation NA, dimension 1/mol).
The Italian scientist Amadeo Avogadro put forward a hypothesis in 1811, which was later confirmed by experimental data and was subsequently called Avogadro's law. He drew attention to the fact that all gases are equally compressed (Boyle-Mariotte's law) and have the same coefficients of thermal expansion (Gay-Lussac's law). In this regard, he suggested that:
equal volumes of different gases under the same conditions contain the same number of molecules.
Under the same conditions (usually we talk about normal conditions: the absolute pressure is 1013 millibars and the temperature is 0 ° C) the distance between the molecules of all gases is the same, and the volume of the molecules is negligible. Considering all of the above, we can make the following assumption:
!if equal volumes of gases under the same conditions contain the same number of molecules, then the masses containing the same number of molecules must have the same volumes.
In other words,
Under the same conditions, 1 mole of any gas occupies the same volume. Under normal conditions, 1 mole of any gas occupies a volume v, equal to 22.4 l. This volume is calledmolar volume of gas (dimension l/mol or m³ /mol).
The exact value of the molar volume of gas under normal conditions (pressure 1013 millibars and temperature 0 ° C) is 22.4135 ± 0.0006 l/mol. Under standard conditions (t=+15° C, pressure = 1013 mbar) 1 mole of gas occupies a volume of 23.6451 liters, and att=+20° C and a pressure of 1013 mbar, 1 mole occupies a volume of about 24.2 liters.
In numerical terms, molar mass coincides with the masses of atoms and molecules (in amu) and with relative atomic and molecular masses.
Consequently, 1 mole of any substance has a mass in grams that is numerically equal to the molecular mass of this substance, expressed in atomic mass units.
For example, M(O2) = 16 a. e.m. 2 = 32 a. e.m., thus, 1 mole of oxygen corresponds to 32 g. The densities of gases measured under the same conditions are referred to as their molar masses. Since when transporting liquefied gases on gas carriers the main object of practical problems are molecular substances (liquids, vapors, gases), the main sought-for quantities will be molar mass M(g/mol), amount of substance v in moles and mass T substances in grams or kilograms.
Knowing the chemical formula of a particular gas, you can solve some practical problems problems arising during the transportation of liquefied gases.
Example 1. A deck tank contains 22 tons of liquefied ethylene (WITH2 N4 ). It is necessary to determine whether there is enough cargo on board to blow through three cargo tanks with a volume of 5000 m 3 each, if after blowing the temperature of the tanks is 0 ° C and the pressure is 1013 millibars.
1. Determine the molecular weight of ethylene:
M = 2 12.011 + 4 1.0079 = 28.054 g/mol.
2. Calculate the density of ethylene vapor under normal conditions:
ρ = M/V = 28.054: 22.4 = 1.232 g/l.
3. Find the volume of cargo vapor under normal conditions:
22∙10 6: 1.252= 27544 m3.
The total volume of cargo tanks is 15,000 m3. Consequently, there is enough cargo on board to purge all cargo tanks with ethylene vapor.
Example 2. It is necessary to determine how much propane (WITH3 N8 ) will be required for purging cargo tanks with a total capacity of 8000 m 3, if the temperature of the tanks is +15 ° C, and the pressure of propane vapor in the tank after the end of purging will not exceed 1013 millibars.
1. Determine the molar mass of propane WITH3 N8
M = 3 12,011 + 8 1,0079 = 44.1 g/mol.
2. Let’s determine the propane vapor density after purging the tanks:
ρ = M: v = 44.1: 23.641 = 1.865 kg/m 3.
3. Knowing the vapor density and volume, we determine the total amount of propane required to purge the tank:
m = ρ v = 1.865 8000 = 14920 kg ≈ 15 t.
>> Mass of an atom. Relative atomic mass
Atomic mass. Relative atomic mass
The material in this paragraph will help you figure out:
> what is the difference between atomic mass and relative atomic mass ;
> why is it convenient to use relative atomic masses;
> where to find the relative atomic mass of an element.
This is interesting
The mass of an electron is approximately 9 10 -28 g.
Atomic mass.
An important characteristic of an atom is its mass. Almost all the mass of an atom is concentrated in the nucleus. Electrons have such a small mass that they are usually neglected.
compared with 1/12 - the mass of a Carbon atom (it is almost 12 times heavier than a Hydrogen atom). This small mass was called the atomic mass unit (abbreviated as a.m.u.):
1 a. e.m. = 1/12m a (C) = 1/12 1.994 10 -23 g = 1.662 10 -24 g.
The mass of the Hydrogen atom almost coincides with the atomic mass unit: m a (H) ~ 1a. e.m. The mass of the Uranium atom is greater than it in
That is
m a (U) ~ 238 a. eat.
The number obtained by dividing the mass of an atom of an element by the atomic mass unit is called the relative atomic mass of the element. This value is denoted by A r (E):

The index next to the letter A is the first letter in the Latin word relativus - relative.
The relative atomic mass of an element shows how many times the mass of an atom element more than 1/12 the mass of a carbon atom.
m a (N) = 1.673 10 -2 4 g
m a (H)= 1 a. eat.
A r (H) = 1
The relative atomic mass of an element has no dimension.
The first table of relative atomic masses was compiled almost 200 years ago by the English scientist J. Dalton.
Based on the material presented, the following conclusions can be drawn:
Relative atomic masses are proportional to the masses of the atoms;
the ratios of atomic masses are the same as the relative atomic masses.
The relative atomic masses of chemical elements are written in periodic table .
John Dalton (1766- 1844)

Outstanding English physicist and chemist. Member of the Royal Society of London (English Academy of Sciences). He was the first to put forward a hypothesis about the different masses and sizes of atoms, determined the relative atomic masses of many elements and compiled the first table of their values (1803). He proposed symbols for elements and designations for chemical compounds.
Having made over 200,000 meteorological observations, having studied the composition and properties of air, he discovered the laws of partial pressures gases(1801), thermal expansion of gases (1802), solubility of gases in liquids (1803).

Rice. 35. Cell of the element Uranus
They are determined with very high accuracy; the corresponding numbers are mostly five and six digits (Fig. 35).
In ordinary chemical calculations, relative atomic masses are usually rounded to whole numbers. So, for Hydrogen and Uranus
A r (H) = 1.0079 ~ 1;
A r (U) = 238.029 ~ 238.
Only the relative atomic mass of Chlorine is rounded to the nearest tenth:
A r (Cl) = 35.453 ~ 35.5.
Find the relative atomic masses of Lithium, Carbon, Oxygen, Neon in the periodic table and round them to whole numbers.
How many times are the masses of the atoms of Carbon, Oxygen, Neon and Magnesium greater than the mass of the Helium atom? For calculations, use rounded values of relative atomic masses.
note: elements are arranged in the periodic table in order of increasing atomic masses.
conclusions
Atoms have extremely low mass.
For convenience of calculations, relative masses of atoms are used.
The relative atomic mass of an element is the ratio of the mass of an atom of the element to the mass of a carbon atom.
The values of relative atomic masses are indicated in the periodic table of chemical elements.
?
48. What is the difference between the concepts “atomic mass” and relative atomic mass?”
49. What is the atomic mass unit?
50. What do the entries A r and A r mean?
51. Which atom is lighter - Carbon or Titan? How many times?
52. Which has more mass: a Fluor atom or two Lithium atoms; two atoms of Magnesium or three atoms of Sulfur?
53. Find in the periodic table three or four pairs of elements whose atomic mass ratio is: a) 1: 2; b) 1:3.
54. Calculate the relative atomic mass of Helium if the mass of an atom of this element is 6.647 - 10 -24 g.
55. Calculate the mass of a Beryllium atom.
Popel P. P., Kryklya L. S., Chemistry: Pidruch. for 7th grade zagalnosvit. navch. closing - K.: VC "Academy", 2008. - 136 p.: ill.
Lesson content lesson outline and supporting frame lesson presentation interactive technologies accelerator teaching methods Practice tests, testing online tasks and exercises homework workshops and trainings questions for class discussions Illustrations video and audio materials photographs, pictures, graphs, tables, diagrams, comics, parables, sayings, crosswords, anecdotes, jokes, quotes Add-ons abstracts cheat sheets tips for the curious articles (MAN) literature basic and additional dictionary of terms Improving textbooks and lessons correcting errors in the textbook, replacing outdated knowledge with new ones Only for teachers calendar plans learning programs guidelinesOne of the fundamental concepts of chemistry is the atomic mass of an element, which is used in almost any chemical calculation. The ability to calculate atomic mass will be useful mainly for schoolchildren and those who plan to study chemistry in the future. However, the formula for calculating atomic mass is incredibly simple.
Definition and Formula
Atomic mass is the sum of the masses of all protons, neutrons and electrons that make up an atom. Compared to the masses of protons and neutrons, the mass of electrons is negligible, so electrons are not taken into account in calculations. Since the mass of neutrons and protons themselves is calculated in infinitesimal numbers to the negative power of 27, for convenience of calculations, relative atomic mass is used, which is expressed in faceless atomic units.
Atomic mass unit- this is a relative value equal to 1/12 of the mass of the carbon-12 nucleus, the nucleus of which contains 6 neutrons and 6 protons. Thus, the formula for determining atomic mass looks like this:
Mass = number of neutrons + number of protons.
Using this formula, the atomic masses of individual isotopes of chemical elements are calculated. This means that the mass of uranium-238 is 238 amu, while uranium-235 has a mass number of 235. This chemical element is generally rich in isotopes, so there are uranium nuclei with mass numbers of 232, 233, 234, 235 , 236 and 238. Despite this diversity, uranium-238 occupies 99% of all uranium in nature, so if you calculate the average value of atomic numbers, the chemical element uranium has an atomic weight of 238.029.
Thus, it is important to understand the difference between atomic mass and average atomic weight:
- atomic mass - the sum of neutrons and protons of a particular isotope (always an integer);
- atomic weight - the arithmetic mean of the atomic masses of all isotopes that occur in nature (usually a fractional number).
Another example
Hydrogen is the most abundant element in the Universe. 99% of hydrogen is protium or hydrogen-1, which contains only 1 proton. There are also isotopes: deuterium or hydrogen-2 and tritium or hydrogen-3. These isotopes have atomic masses of 2 and 3, respectively, but they are extremely rare in nature, so the atomic weight of hydrogen is 1.00784.
Finding atomic mass
You can determine the atomic number for a selected element using the periodic table. The element number in the table always matches the number of protons in the nucleus. For example, the hydrogen mentioned above has the first number in the table and contains only 1 proton. The table below always shows the average atomic weight of an element, which must be rounded to the nearest whole number for calculations.
Initially displays all the information on the number of protons and electrons in an atom, as well as its atomic mass. That is why in school tasks To determine atomic mass, it is enough to use the periodic table and not do any special calculations.
Usually in chemistry lessons the inverse problem is posed: how to determine the number of neutrons in a particular isotope? In this case, a simple formula applies:
Number of neutrons = atomic mass – atomic number.
For example, the hydrogen atom-1 does not contain neutrons, since its atomic number is also equal to one. But tritium is already hydrogen with one proton and two neutrons. Tritium is an unstable isotope. It easily decays into helium atoms, free electrons and antineutrinos, releasing a certain amount of energy. Unstable isotopes are called radioactive.
Let's look at an example
Determination of atomic mass
Let's consider oxygen - a chemical element that has an atomic number of 8 periodic table Mendeleev. This means that oxygen has 8 protons in its nucleus, as well as 8 electrons in its orbits. The atomic mass shown in the table is 16 a. e. m, to calculate which we do not need a calculator. From this information we can determine that an oxygen atom contains 8 neutrons. However, the number of neutrons can easily change depending on external conditions.
If oxygen loses or gains one neutron, we get a new isotope whose atomic mass changes. Using a calculator, you can calculate the mass numbers of different isotopes of oxygen, which, however, contain the answer to this question in their very name. In nature, there are 3 stable isotopes of oxygen: oxygen-16, oxygen-17 and oxygen-18. The last two have “extra” neutrons in the nucleus.
In addition, there are unstable isotopes of oxygen, whose half-lives range from a few minutes to millionths of nanoseconds.
Conclusion
Mass number is an important parameter of any element, with which it is calculated molar masses when conducting chemical reactions. However, the mass number is always indicated in the periodic table of Mendeleev, so our calculator will be useful mainly for schoolchildren who are just beginning to study the amazing science of chemistry.
Absolute masses of atoms One of the fundamental properties of atoms is their mass. Absolute (true) mass of an atom– the value is extremely small. It is impossible to weigh atoms on a balance because such precise scales do not exist. Their masses were determined using calculations. For example, the mass of one hydrogen atom is 0.000 000 000 000 000 000 000 001 663 grams! The mass of a uranium atom, one of the heaviest atoms, is approximately 0.000 000 000 000 000 000 000 4 grams. Writing and reading these numbers is not easy; You can make a mistake by missing a zero or adding an extra one. There is another way to write it - in the form of a product: 4 ∙ 10−22 (22 is the number of zeros in the previous number). The exact mass of the uranium atom is 3.952 ∙ 10−22 g, and the hydrogen atom, the lightest among all atoms, is 1.673 ∙ 10−24 g. It is inconvenient to carry out calculations with small numbers. Therefore, instead of the absolute masses of atoms, their relative masses are used.
Relative atomic mass
The mass of any atom can be judged by comparing it with the mass of another atom (find the ratio of their masses). Since the determination of the relative atomic masses of elements, various atoms as a comparison. At one time, hydrogen and oxygen atoms were unique standards for comparison. A unified scale of relative atomic masses and a new unit of atomic mass, adopted International Congress of Physicists (1960) and unified by the International Congress of Chemists (1961). To this day, the standard for comparison is 1/12 of the mass of a carbon atom. This value is called the atomic mass unit, abbreviated a.u.m. Atomic mass unit (amu) – mass of 1/12 of a carbon atom Let's compare how many times the absolute mass of a hydrogen and uranium atom differs from 1 amu, to do this we divide these numbers by one another: The values obtained in the calculations are the relative atomic masses of the elements - relative to 1/12 the mass of a carbon atom. Thus, the relative atomic mass of hydrogen is approximately 1, and that of uranium is 238. Please note that relative atomic mass has no units, since absolute mass units (grams) are canceled out when dividing. The relative atomic masses of all elements are indicated in the Periodic Table of Chemical Elements by D.I. Mendeleev. The symbol used to indicate relative atomic mass is Аr (the letter r is an abbreviation for the word relative, which means relative). The relative atomic masses of elements are used in many calculations. As a rule, values given in the Periodic Table are rounded to whole numbers. Note that the elements in the Periodic Table are arranged in order of increasing relative atomic masses. For example, using Periodic System Let's determine the relative atomic masses of a number of elements:Ar(O) = 16; Ar(Na) = 23; Ar(P) = 31. The relative atomic mass of chlorine is usually written as 35.5! Ar(Cl) = 35.5
- Relative atomic masses are proportional to the absolute masses of atoms
- The standard for determining relative atomic mass is 1/12 of the mass of a carbon atom
- 1 amu = 1.662 ∙ 10−24 g
- Relative atomic mass is denoted by Ar
- For calculations, the values of relative atomic masses are rounded to whole numbers, with the exception of chlorine, for which Ar = 35.5
- Relative atomic mass has no units of measurement
Currently, the atomic mass unit is taken to be equal to 1/12 the mass of a neutral atom of the most common isotope of carbon 12 C, so the atomic mass of this isotope by definition is exactly 12. The difference between the atomic mass of an isotope and its mass number is called excess mass (usually expressed in MeV ). It can be either positive or negative; the reason for its occurrence is the nonlinear dependence of the binding energy of nuclei on the number of protons and neutrons, as well as the difference in the masses of the proton and neutron.
The dependence of the atomic mass of an isotope on the mass number is as follows: the excess mass is positive for hydrogen-1, with increasing mass number it decreases and becomes negative until a minimum is reached for iron-56, then it begins to grow and increases to positive values for heavy nuclides. This corresponds to the fact that the fission of nuclei heavier than iron releases energy, while the fission of light nuclei requires energy. On the contrary, the fusion of nuclei lighter than iron releases energy, while the fusion of elements heavier than iron requires additional energy.
Story
Until the 1960s, atomic mass was defined so that the nuclide oxygen-16 had an atomic mass of 16 (oxygen scale). However, the ratio of oxygen-17 to oxygen-18 in natural oxygen, which was also used in atomic mass calculations, resulted in the presence of two different tables atomic masses. Chemists used a scale based on the fact that the natural mixture of oxygen isotopes would have an atomic mass of 16, while physicists assigned the same number of 16 to the atomic mass of the most common isotope of oxygen (which has eight protons and eight neutrons).
Links
Wikimedia Foundation.
2010.
See what “Atomic mass” is in other dictionaries: The mass of an atom, expressed in atomic mass units. Atomic mass is less than the sum of the masses of the particles that make up the atom (protons, neutrons, electrons) by an amount determined by the energy of their interaction (see, for example, Mass Defect) ...
Big Encyclopedic Dictionary Atomic mass atomic mass chemical element , expressed in atomic mass units (amu). For 1 amu 1/12 of the mass of the carbon isotope with atomic mass 12 is accepted. 1 amu = 1.6605655 10 27 kg. The atomic mass consists of the masses of all protons and...
Nuclear energy terms atomic mass - is the mass of atoms of an element, expressed in atomic mass units. The mass of an element that contains the same number of atoms as 12 g of the isotope 12C. general chemistry : textbook / A. V. Zholnin ...
Chemical terms ATOMIC MASS - dimensionless quantity. A. m. mass of an atom chemical. element expressed in atomic units (see) ...
Big Polytechnic Encyclopedia - (obsolete term atomic weight), the relative value of the mass of an atom, expressed in atomic mass units (a.m.u.). A.m. is less than the sum of the masses of the constituent atoms per mass defect. A. m. was taken by D. I. Mendeleev as the basis. characteristic of the element when... ...
Nuclear energy terms Physical encyclopedia - - [Ya.N.Luginsky, M.S.Fezi Zhilinskaya, Yu.S.Kabirov. English-Russian dictionary of electrical engineering and power engineering, Moscow, 1999] Topics of electrical engineering, basic concepts EN atomic weight ...
Technical Translator's Guide The mass of an atom, expressed in atomic mass units. The atomic mass of a chemical element consisting of a mixture of isotopes is taken to be the average value of the atomic mass of isotopes, taking into account their percentage content (this value is given in periodic... ...
The concept of this quantity has undergone long-term changes in accordance with changes in the concept of atoms. According to Dalton's theory (1803), all atoms of the same chemical element are identical and its atomic mass is a number equal to... ... Collier's Encyclopedia
Nuclear energy terms- santykinė atominė masė statusas T sritis Standartizacija ir metrologija apibrėžtis Cheminio elemento vidutinės masės ir nuklido ¹²C atomo masės 1/12 dalies dalmuo. atitikmenys: engl. atomic mass; atomic weight; relative atomic mass vok. Atommasse…
Nuclear energy terms- santykinė atominė masė statusas T sritis Standartizacija ir metrologija apibrėžtis Vidutinės elemento atomų masės ir 1/12 nuklido ¹²C atomo masės dalmuo. atitikmenys: engl. atomic mass; atomic weight; relative atomic mass vok. Atommasse, f;… … Penkiakalbis aiškinamasis metrologijos terminų žodynas