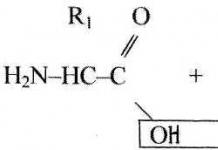
The content of the article
NUCLEIC ACIDS- biological polymer molecules that store all the information about an individual living organism, determining its growth and development, as well as hereditary traits transmitted to the next generation. Nucleic acids are found in the nuclei of cells of all plant and animal organisms, which determined their name (lat. . nucleus - core).
The composition of the polymer chain of nucleic acids.
polymer chain nucleic acids assembled from fragments of phosphoric acid H 3 PO 3 and fragments of heterocyclic molecules, which are derivatives of furan. There are only two types of nucleic acids, each built on the basis of one of the two types of such heterocycles - ribose or deoxyribose (Fig. 1).
Rice. one. STRUCTURE OF RIBOSE AND DEOXYRIBOSE.
The name ribose (from lat. . Rib - rib, paper clip) has an ending - ose, which indicates belonging to the class of sugars (for example, glucose, fructose). The second compound does not have an OH group (hydroxy group), which is marked in red in ribose. In this regard, the triple compound is called deoxyribose, i.e., ribose devoid of an oxy group.
The polymer chain, built from fragments of ribose and phosphoric acid, is the basis of one of the nucleic acids - ribonucleic acid (RNA). The term "acid" in the name of this compound is used because one of the acidic OH groups of phosphoric acid remains unsubstituted, which gives the whole compound a slightly acidic character. If deoxyribose is involved in the formation of the polymer chain instead of ribose, then deoxyribonucleic acid is formed, for which the well-known abbreviation DNA is commonly accepted.
Structure of DNA.
The DNA molecule serves as the starting point in the process of growth and development of an organism. On fig. Figure 2 shows how two types of alternating initial compounds are combined into a polymer chain; not a synthesis method is shown, but a schematic diagram of the assembly of a DNA molecule.

In the final version, the polymeric DNA molecule contains nitrogen-containing heterocycles in the lateral frame. Four types of such compounds are involved in the formation of DNA, two of them are six-membered rings, and two are fused rings, where the six-membered ring is soldered to the five-membered one (Fig. 3).

Rice. 3. STRUCTURE OF NITROGEN-CONTAINING HETEROCYCLES that make up DNA
At the second stage of assembly, the nitrogen-containing heterocyclic compounds shown above are added to the free OH groups of deoxyribose, forming side pendants in the polymer chain (Fig. 4).

The molecules of adenine, thymine, guanine and cytosine attached to the polymer chain are designated by the first letters of the names of the initial compounds, that is, AND, T, G and C.
The DNA polymer chain itself has a certain direction - when mentally moving along the molecule in the forward and reverse direction, the same groups that make up the chain meet on the way in a different sequence. When moving in one direction from one phosphorus atom to another, at first the CH 2 group goes along the route, and then two CH groups (oxygen atoms can be ignored), when moving in the opposite direction, the sequence of these groups will be reversed (Fig. 5) .

Rice. five. DIRECTIONALITY OF THE DNA POLYMER CHAIN. When describing the order in which the attached heterocycles alternate, it is customary to use the direct direction, that is, from the CH 2 group to the CH groups.
The very concept of “strand direction” helps to understand how two strands of DNA are arranged when they are combined, and is also directly related to protein synthesis.
At the next stage, two DNA molecules are combined, arranged in such a way that the beginning and ends of the chains are directed towards opposite sides. In this case, the heterocycles of the two chains face each other and turn out to be arranged in some optimal way, meaning that hydrogen bonds arise between the pairs of С=О and NH 2 groups, as well as between є N and NH=, which are part of the heterocycles ( cm. HYDROGEN BOND). On fig. Figure 6 shows how the two chains are arranged relative to each other and how hydrogen bonds between heterocycles occur. The most important detail– consists in the fact that the pairs connected by hydrogen bonds are rigidly defined: the fragment AND always interact with T, and the fragment G- always with C. The strictly defined geometry of these groups leads to the fact that these pairs fit each other extremely precisely (like a key to a lock), a pair A-T linked by two hydrogen bonds, and the pair G-C- three links.

Hydrogen bonds noticeably weaker than conventional valence bonds, but due to their large number along the entire polymer molecule, the connection of two chains becomes quite strong. The DNA molecule contains tens of thousands of groups AND, T, G and C and the order of their alternation within one polymer molecule may be different, for example, in a certain section of the chain, the sequence may look like: - AND-AND-T-G-C-G-AND-T-. Since the interacting groups are strictly defined, then on the opposite site of the second polymer molecule there will necessarily be a sequence - T-T-AND-C-G-C-T-AND-. Thus, knowing the arrangement of heterocycles in one chain, one can indicate their placement in another chain. It follows from this correspondence that the total number of groups in a doubled DNA molecule AND equal to the number of groups T, and the number of groups G- quantity C(E.Chargaff's rule).
Two DNA molecules linked by hydrogen bonds are shown in Fig. 5 in the form of two flat chains, but in reality they are located in a different way. The true direction in space of all bonds, determined by bond angles and contracting hydrogen interactions, leads to certain bending of polymer chains and rotation of the plane of heterocycles, which is approximately shown in the first video clip of Fig. 7 using structural formula. Much more accurately, the entire spatial structure can be conveyed only with the help of three-dimensional models (Fig. 7, second video fragment). In this case, a complex picture arises, therefore it is customary to use simplified images, which are especially widely used when depicting the structure of nucleic acids or proteins. In the case of nucleic acids, polymer chains are depicted in the form of flat ribbons, and heterocyclic groups AND, T, G and C– in the form of lateral rods or simple valence strokes having different colors, or containing letter designations of the corresponding heterocycles at the end (Fig. 7, third video fragment).

During the rotation of the entire structure around the vertical axis (Fig. 8), the helical shape of two polymer molecules is clearly visible, which are, as it were, wound on the surface of the cylinder, this is the well-known DNA double helix.

With such a simplified image, the main information does not disappear - the order of alternation of the grouping AND, T, G and C, which determines the individuality of each living organism, all information is written in a four-letter code.
The structure of the polymer chain and the obligatory presence of four types of heterocycles are the same for all representatives of the living world. In all animals and higher plants, the number of pairs AND – T always somewhat more than steam G – C. The difference between mammalian DNA and plant DNA is that mammals have a pair AND – T throughout the chain is slightly more common (approximately 1.2 times) than a pair G – C. In the case of plants, the preference for the first pair is much more noticeable (approximately 1.6 times).
DNA is one of the largest polymeric molecules known today, with hundreds of millions of links in some organisms. The length of such a molecule reaches several centimeters, which is a very large value for molecular objects. Because cross section molecules are only 2 nm (1nm = 10 -9 m), then its proportions can be compared with a railway rail tens of kilometers long.
Chemical properties of DNA.
In water, DNA forms viscous solutions; when such solutions are heated to 60 ° C or under the action of alkalis, the double helix breaks up into two component chains, which can again be combined if you return to the original conditions. Under slightly acidic conditions, hydrolysis occurs, as a result, fragments -P-O-CH 2 - are partially cleaved with the formation of fragments -P-OH and HO-CH 2 , respectively, as a result, monomeric, dimeric (double) or trimeric (triple) acids are formed, which are links from which the DNA chain was assembled (Fig. 9).
Rice. nine. FRAGMENTS OBTAINED WHEN CLEAVING DNA.
Deeper hydrolysis makes it possible to separate deoxyribose sites from phosphoric acid, as well as the grouping G from deoxyribose, i.e., to disassemble the DNA molecule into its constituent components in more detail. Under action strong acids(in addition to the decay of fragments -P (O) -O-CH 2 -) groups are also split off AND and G. The action of other reagents (for example, hydrazine) makes it possible to separate groups T and C. A more delicate splitting of DNA into components is carried out using a biological preparation - deoxyribonuclease, isolated from the pancreas (end - aza always indicates that the substance is a catalyst of biological origin - an enzyme). The initial part of the name - deoxyribonuclease- indicates which compound is cleaved by this enzyme. All of these methods of DNA cleavage are focused primarily on a detailed analysis of its composition.
The most important information, contained in the DNA molecule, is the order of alternation of groups AND, T, G and C, it is obtained using specially developed techniques. For this, a wide range of enzymes have been created that find a strictly defined sequence in the DNA molecule, for example, C-T-G-C-A-G(as well as the sequence corresponding to it on the opposite strand G-AND-C-G-T-C) and isolate it from the chain. The enzyme Pst I has this property (trade name, it is formed from the name of that microorganism P rovidencia st uartii, from which this enzyme is derived). When using another Pal I enzyme, it is possible to find the sequence G-G-C-C. Next, the results obtained with the action of a wide range of different enzymes according to a predetermined scheme are compared, as a result, it is possible to determine the sequence of such groups in a certain DNA region. Now such techniques have been brought to the stage of widespread use, they are used in a wide variety of areas far from scientific biochemical research, for example, in identifying the remains of living organisms or establishing the degree of relationship.
RNA structure
in many ways resembles DNA, the difference is that in the main chain, fragments of phosphoric acid alternate with ribose, and not with deoxyribose (Fig.). The second difference is that the uracil heterocycle ( At) instead of thymine ( T), other heterocycles AND, G and C the same as DNA. Uracil differs from thymine in the absence of a methyl group attached to the ring, in Fig. 10 this methyl group is highlighted in red.

Rice. 10. THE DIFFERENCE OF THYMIN FROM URAcil– the absence of a methyl group in the second compound, highlighted in red in thymine.
A fragment of an RNA molecule is shown in fig. 11, grouping order AND, At, G and C, as well as their quantitative ratio may be different.

Fig.11. RNA MOLECULE FRAGMENT. The main difference from DNA is the presence of OH groups in ribose (red) and a uracil fragment (blue).
The polymeric chain of RNA is about ten times shorter than that of DNA. An additional difference is that RNA molecules do not combine into double helixes consisting of two molecules, but usually exist as a single molecule, which in some areas can form double-stranded helical fragments with itself, alternating with linear sections. On helical sections, the interaction of pairs is observed as strictly as in DNA. Pairs linked by hydrogen bonds and forming a helix ( AND-At and G-C), appear in those areas where the location of the groups is favorable for such an interaction (Fig. 12).

For the vast majority of living organisms, the quantitative content of pairs AND-At more than G-C, in mammals by 1.5–1.6 times, in plants by 1.2 times. There are several types of RNA, the roles of which are different in a living organism.
Chemical properties of RNA
resemble the properties of DNA, but the presence additional groups OH in ribose and a lower (in comparison with DNA) content of stabilized helical regions makes RNA molecules chemically more vulnerable. Under the action of acids or alkalis, the main fragments of the P(O)-O-CH 2 polymer chain are easily hydrolyzed, groups AND, At, G and C split off more easily. If you want to get monomeric fragments (like those in Fig. 9), while retaining chemically bound heterocycles, delicately acting enzymes called ribonculases are used.
Participation of DNA and RNA in protein synthesis
is one of the main functions of nucleic acids. Proteins are the most important components of every living organism. Muscles, internal organs, bone tissue, skin and hair in mammals are composed of proteins. These are polymeric compounds that are assembled in a living organism from various amino acids. In such an assembly, nucleic acids play a controlling role, the process takes place in two stages, and in each of them the determining factor is the mutual orientation of nitrogen-containing heterocycles of DNA and RNA.
The main task of DNA is to store recorded information and provide it at the moment when protein synthesis begins. In this regard, the increased chemical stability of DNA in comparison with RNA is understandable. Nature has taken care to keep the basic information intact as much as possible.
At the first stage, part of the double helix opens, the liberated branches diverge, and on groups AND, T, G and C available, the synthesis of RNA, called messenger RNA, begins, since it, as a copy from the matrix, accurately reproduces the information recorded on the uncovered DNA section. Opposite the group AND, belonging to the DNA molecule, there is a fragment of the future messenger RNA containing the group At, all other groups are located opposite each other in exact accordance with how it occurs during the formation of the DNA double helix (Fig. 13).

According to this scheme, a polymeric messenger RNA molecule containing several thousand monomeric units is formed.
At the second stage, template DNA moves from the cell nucleus to the perinuclear space - the cytoplasm. The so-called transfer RNAs, which carry (transport) various amino acids, are suitable for the resulting messenger RNA. Each transfer RNA, loaded with a specific amino acid, approaches a strictly conditioned region of messenger RNA, Right place is detected using the same principle of mutual correspondence of groups AND
An important detail is that the temporal interaction of messenger and transfer RNA goes through only three groups, for example, to the triad C-C-At matrix acid, only the corresponding triple G-G-AND transfer RNA, which certainly carries with it the amino acid glycine (Fig. 14). Likewise for the triad G-AND-At only a set can approach C-At-AND transporting only the amino acid leucine. Thus, the sequence of groups in messenger RNA indicates in what order the amino acids should be connected. In addition, the system contains additional regulatory rules in coded form, some sequences from the three groups of messenger RNA indicate that protein synthesis should stop at this point, i.e. the molecule has reached the required length.
Shown in fig. 14 protein synthesis takes place with the participation of another - the third type of RNA acids, they are part of the ribosomes and therefore they are called ribosomal. The ribosome, which is an ensemble of certain proteins of ribosomal RNA, ensures the interaction of messenger and transfer RNA, playing the role of a conveyor belt that moves the messenger RNA one step after the connection of two amino acids has occurred.
The main meaning of the two-stage scheme shown in Fig. 13 and 14 consists in the fact that the polymer chain of a protein molecule is assembled from various amino acids in the intended order and strictly according to the plan that was encoded in a certain DNA section. So DNA is the starting point of this whole programmed process.
In the process of life, proteins are constantly consumed, and therefore they are regularly reproduced according to the described scheme, the entire synthesis of a protein molecule, consisting of hundreds of amino acids, takes place in a living organism for approximately one minute.
The first studies of nucleic acids were carried out in the second half of the 19th century, the understanding that all information about a living organism is encrypted in DNA came in the middle of the 20th century, the structure of the DNA double helix was established in 1953 by J. Watson and F. Crick based on the data X-ray diffraction analysis, which is recognized as the largest scientific achievement 20th century. In the mid 70s of the 20th century. methods for deciphering the detailed structure of nucleic acids appeared, and then methods for their targeted synthesis were developed. Today, far from all the processes occurring in living organisms with the participation of nucleic acids are clear, and today this is one of the most intensively developing areas of science.
Mikhail Levitsky
Ministry of Education and Science of the Russian Federation
federal state autonomous educational institution
« KAZAN NATIONAL RESEARCH TECHNOLOGICAL UNIVERSITY»
INSTITUTE OF FOOD ENGINEERING
DEPARTMENT OF FOOD BIOTECHNOLOGY
SUMMARY ON THE TOPIC
NUCLEIC ACIDS. DNA and RNA
Completed by: Radenko V.
Group 625 M-52
Nucleic acids - natural high-molecular organic compounds that ensure the storage and transmission of hereditary (genetic) information in living organisms. There are 2 types of nucleic acids in every living organism: ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). The molecular weight of the "smallest" known nucleic acid, transfer RNA (tRNA), is approximately 25 kD. DNA is the largest polymer molecules; their molecular weight varies from 1,000 to 1,000,000 kD. DNA and RNA consist of monomeric units - nucleotides, therefore nucleic acids are called polynucleotides.
The structure of nucleotides
Each nucleotide contains 3 chemically distinct components: heterocyclic nitrogenous base, a monosaccharide (pentose) and a phosphoric acid residue. Depending on the number of phosphoric acid residues present in the molecule, nucleoside monophosphates (NMP), nucleoside diphosphates (NDP), nucleoside triphosphates (NTP) are distinguished (Fig. 4-1). Nucleic acids are composed of two types of nitrogenous bases: purine - adenine(AND), guanine(G) and pyrimidine - cytosine(WITH), thymine(T) and uracil(U). The numbering of atoms in the bases is written inside the cycle (Fig. 4-2). Pentoses in nucleotides are either ribose (in RNA) or deoxyribose (in DNA). To distinguish the numbers of atoms in pentoses from the numbering of atoms in the bases, the entry is made on the outside of the cycle and a stroke (") - 1", 2", 3", 4" and 5" is added to the number (Fig. 4-3). The pentose connects to the base N-glycosidic bond, formed by C 1 atom of pentose (ribose or deoxyribose) and N 1 atom of pyrimidine or N 9 atom of purine (Fig. 4-4). Nucleotides in which pentose is represented by ribose are called ribonucleotides, and nucleic acids built from ribonucleotides are called ribonucleic acids, or RNA. Nucleic acids containing deoxyribose as monomers are called deoxyribonucleic acids, or DNA. Nucleic acids are structurally classified as
Rice. 4-1. Nucleoside mono-, di- and triphosphates of adenosine. Nucleotides are phosphate esters of nucleosides. The phosphoric acid residue is attached to the 5" carbon atom of the pentose (5" phosphoester bond).

Rice. 4-2. Purine and pyrimidine bases.

Rice. 4-3. Pentoses. There are 2 types - β-D-ribose as part of RNA nucleotides and β-D-2-deoxyribose as part of DNA nucleotides.
class of linear polymers. The nucleic acid backbone has the same structure along the entire length of the molecule and consists of alternating groups - pentose-phosphate-pentose- (Fig. 4-5). Variable groups in polynucleotide chains are nitrogenous bases - purines and pyrimidines. RNA molecules include adenine (A), uracil (U), guanine (G) and cytosine (C), DNA - adenine (A), thymine (T), guanine (G) and cytosine (C). The uniqueness of the structure and functional individuality of DNA and RNA molecules are determined by their primary structure - the sequence of nitrogenous bases in the polynucleotide chain.

Rice. 4-4. Purine and pyrimidine nucleotides.

Rice. 4-5. Fragment of a DNA chain.
B. Structure of deoxyribonucleic acid (DNA)
Primary Structure DNA - the order of alternation of deoxyribonucleoside monophosphates (dNMP) in a polynucleotide chain. Each phosphate group in the polynucleotide chain, with the exception of the phosphorus residue at the 5 "-end of the molecule, participates in the formation of two ester bonds involving 3"- and 5"-carbon atoms of two neighboring deoxyriboses, therefore the bond between monomers is denoted by 3", 5"- phosphodiester. The terminal nucleotides of DNA are distinguished by structure: there is a phosphate group at the 5 "end, and a free OH group at the 3" end of the chain. These ends are called 5 "- and 3"-ends. The linear sequence of deoxyribonucleotides in the DNA polymer chain usually abbreviated using a one-letter code, for example -A-G-C-T-T-A-C-A- from 5 "- to 3"-end.
Each nucleic acid monomer contains a phosphoric acid residue. At pH 7, the phosphate group is fully ionized, so in vivo Nucleic acids exist as polyanions (have multiple negative charges). Residues of pentoses also exhibit hydrophilic properties. Nitrogenous bases are almost insoluble in water, but some atoms of the purine and pyrimidine rings are able to form hydrogen bonds.
Secondary structure of DNA. In 1953, J. Watson and F. Crick proposed a model of the spatial structure of DNA. According to this model, the DNA molecule has the shape of a helix formed by two polynucleotide chains twisted relative to each other and around a common axis. double helix right-handed, polynucleotide chain in it antiparallel(Fig. 4-6), i.e. if one of them is oriented in the direction 3"→5", then the second one is oriented in the direction 5"→3". Therefore, at each end
Rice. 4-6. Double helix of DNA.
DNA molecules consist of two antiparallel strands with a complementary nucpeotide sequence. The chains are twisted relative to each other in a right-handed helix so that there are approximately 10 pairs of nucleotides per turn of the molecule. All bases of DNA chains are located inside the double helix, and the pentose phosphate backbone is outside. Polynucleotide chains are held relative to each other due to hydrogen bonds between complementary purine and pyrimidine nitrogenous bases A and T (two bonds) and between G and C (three bonds) (Fig. 4-7). With this combination, each pair contains three rings, so the total size of these base pairs is the same along the entire length of the molecule.

Rice. 4-7. Purine-pyrimidine base pairs in DNA.
Hydrogen bonds with other combinations of bases in a pair are possible, but they are much weaker. The nucleotide sequence of one chain is completely complementary to the nucleotide sequence of the second chain. Therefore, according to the Chargaff rule (Erwin Chargaff in 1951 established patterns in the ratio of purine and pyrimidine bases in a DNA molecule), the number of purine bases (A + G) is equal to the number of pyrimidine bases (T + C). Complementary bases are stacked at the core of the helix. Between the bases of a double-stranded molecule in a stack, hydrophobic interactions, stabilizing the double helix.
Such a structure excludes the contact of nitrogenous residues with water, but the base stack cannot be absolutely vertical. The base pairs are slightly offset from each other. In the formed structure, two grooves are distinguished - a large one, 2.2 nm wide, and a small one, 1.2 nm wide. Nitrogenous bases in the region of the major and minor grooves interact with specific proteins involved in the organization of the chromatin structure.
Tertiary structure of DNA (DNA supercoiling) Each DNA molecule is packaged into a separate chromosome. Diploid human cells contain 46 chromosomes. The total length of the DNA of all the chromosomes of a cell is 1.74 m, but it is packed in a nucleus that is millions of times smaller in diameter. In order to arrange DNA in the nucleus of a cell, a very compact structure must be formed. Compaction and supercoiling of DNA are carried out using a variety of proteins that interact with certain sequences in the DNA structure. All eukaryotic DNA-binding proteins can be divided into 2 groups: histone and nonhistone proteins. The complex of proteins with the nuclear DNA of cells is called chromatin.
Histones- proteins with molecular weight 11-21 kD, containing many residues of arginine and lysine. Due to the positive charge, histones form ionic bonds with negatively charged phosphate groups located on the outside of the DNA double helix. There are 5 types of histones. Four histones H2A, H2B, H3 and H4 form an octameric protein complex (H2A, H2B, H3, H4) 2, which is called "nucleosomal core"(from English. nucleosome core). The DNA molecule "winds" on the surface of the histone octamer, making 1.75 turns (about 146 pairs of nucleotides). Such a complex of histone proteins with DNA serves as the main structural unit of chromatin, it is called "nucleosome". DNA that binds nucleosome particles is called linker DNA. On average, linker DNA is 60 base pairs. Histone H1 molecules bind to DNA in internucleosomal regions (linker sequences) and protect these regions from the action of nucleases (Fig. 4-8).
 Rice. 4-8. Structure of nucleosomes.
Rice. 4-8. Structure of nucleosomes.
Eight histone molecules (H2A, H2B, H3, H4) 2 make up the core of the nucleosome, around which DNA forms approximately 1.75 turns. DNA. Amino acid residues of lysine, arginine and terminal amino groups of histones can be modified: acetylated, phosphorylated, methylated, or interact with the ubiquitin protein (non-histone protein). Modifications are reversible and irreversible, they change the charge and conformation of histones, and this affects the interaction of histones with each other and with DNA. The activity of enzymes responsible for modification is regulated and depends on the stage of the cell cycle. Modifications make possible conformational rearrangements of chromatin.
Non-histone proteins of chromatin. The nucleus of a eukaryotic cell contains hundreds of a wide variety of DNA-binding non-histone proteins. Each protein is complementary to a specific sequence of DNA nucleotides. (DNA site). This group includes the family of site-specific zinc finger proteins (see Section 1). Each " zinc finger"recognizes a specific site, consisting of 5 nucleotide pairs. Another family of site-specific proteins is homodimers. A fragment of such a protein that contacts DNA has a helix-turn-helix structure (see section 1). To the group of structural and regulatory proteins , which are permanently associated with chromatin, include high mobility proteins ( HMG proteins- from English, high mobility gel proteins). They have a molecular weight of less than 30 kD and are characterized by a high content of charged amino acids. Due to their low molecular weight, HMG proteins are highly mobile during polyacrylamide gel electrophoresis. Non-histone proteins also include the enzymes of replication, transcription, and repair. With the participation of structural, regulatory proteins and enzymes involved in the synthesis of DNA and RNA, the nucleosome thread is converted into a highly condensed complex of proteins and DNA. The resulting structure is 10,000 times shorter than the original DNA molecule.
There are three main macromolecules in a living organism: proteins and nucleic acids of two types. Thanks to them, vital activity and the proper functioning of the whole organism are supported. What are nucleic acids? Why are they needed? More on this later in the article.
general information
Nucleic acid is a biopolymer, an organic compound with high molecular weight, which is formed by nucleotide residues. The transmission from generation to generation of all genetic information - the main task performed by nucleic acids. The presentation below will explain this concept in more detail.
Research History
The first nucleotide studied was isolated from the muscles of a bull in 1847 and named "inosinic acid". As a result of studying the chemical structure, it was revealed that it is a riboside-5'-phosphate and stores an N-glycosidic bond. In 1868, a substance called "nuclein" was discovered. It was discovered by the Swiss chemist Friedrich Miescher during the research of some biological substances. The composition of this substance included phosphorus. The connection had acid properties and was not subjected to decomposition under the influence of proteolytic enzymes.

The substance received the formula C29H49N9O22P3. The assumption of the participation of nuclein in the process of transmission of hereditary information was put forward as a result of the discovery of its similarity chemical composition with chromatin. This element is the main component of chromosomes. The term "nucleic acid" was first introduced in 1889 by Richard Altmann. It was he who became the author of a method for obtaining these substances without protein impurities. During the study of alkaline hydrolysis of nucleic acids, Levin and Jacob identified the main components of the products of this process. They were nucleotides and nucleosides. In 1921, Lewin suggested that DNA has a tetranucleotide structure. However, this hypothesis was not confirmed and turned out to be erroneous.

Classification
Nucleic acids are of two types: DNA and RNA. Their presence is found in the cells of all living organisms. DNA is mainly found in the nucleus of the cell. RNA is found in the cytoplasm. In 1935, during the soft fragmentation of DNA, 4 DNA-forming nucleotides were obtained. These components are presented in the state of crystals. In 1953, Watstone and Crick determined that DNA has a double helix.
Extraction methods

Developed various ways obtaining compounds from natural sources. The main conditions of these methods are effective separation of nucleic acids and proteins, the least fragmentation of substances obtained during the process. To date, the classical method is widely used. The essence of this method is the destruction of the walls of biological material and their further processing with an anionic detergent. The result is a precipitate of the protein, and the nucleic acids remain in solution. Another method is also used. In this case, nucleic acids can be gelled using ethanol and saline. In doing so, some care must be taken. In particular, ethanol must be added with great care in saline solution to obtain a gel precipitate. At what concentration the nucleic acid was isolated, what impurities are present in it, can be determined by the spectrophotometric method. Nucleic acids are easily degraded by nuclease, which is a special class of enzymes. With such a release, it is necessary that laboratory equipment undergo mandatory treatment with inhibitors. These include, for example, a DEPC inhibitor, which is used in RNA isolation.
Physical Properties
Nucleic acids have good solubility in water, and in organic compounds almost insoluble. In addition, they are particularly sensitive to temperature and pH levels. High molecular weight nucleic acid molecules can be fragmented by nuclease under the influence of mechanical forces. These include mixing the solution, shaking it.
Nucleic acids. Structure and functions

Polymeric and monomeric forms of the compounds under consideration are found in cells. The polymeric forms are called polynucleotides. In this form, chains of nucleotides are linked by a phosphoric acid residue. Due to the content of two types of heterocyclic molecules called ribose and deoxoriboose, acids, respectively, are ribonucleic and deoxyribonucleic. With their help, the storage, transmission and implementation of hereditary information takes place. Of the monomeric forms of nucleic acids, adenosine triphosphoric acid is the most popular. It is involved in signaling and providing energy reserves in the cell.
DNA
Deoxyribonucleic acid is a macromolecule. With its help, the process of transmission and implementation of genetic information takes place. This information is necessary for the program of development and functioning of a living organism. In animals, plants, fungi, DNA is part of the chromosomes located in the nucleus of the cell, and is also found in mitochondria and plastids. In bacteria and archaea, the deoxyribonucleic acid molecule clings to the cell membrane from the inside. In such organisms, mainly circular DNA molecules are present. They are called "plasmids". According to the chemical structure, deoxyribonucleic acid is a polymer molecule consisting of nucleotides. These components, in turn, are composed of a nitrogenous base, sugar and a phosphate group. It is due to the last two elements that a bond is formed between nucleotides, creating chains. Basically, the DNA macromolecule is presented in the form of a helix of two chains.

RNA
Ribonucleic acid is a long chain of nucleotides. They contain a nitrogenous base, ribose sugar and a phosphate group. Genetic information is encoded using a sequence of nucleotides. RNA is used to program protein synthesis. Ribonucleic acid is created during transcription. This is the process of RNA synthesis on a DNA template. It occurs with the participation of special enzymes. They are called RNA polymerases. After that, matrix ribonucleic acids participate in the translation process. This is how protein synthesis takes place on an RNA template. Ribosomes take an active part in this process. The remaining RNAs undergo chemical transformations at the end of transcription. As a result of the ongoing changes, secondary and tertiary structures of ribonucleic acid are formed. They function depending on the type of RNA.