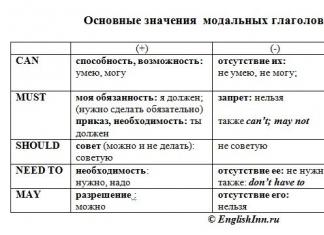
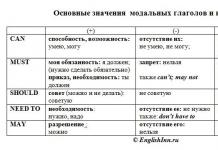
Alkenes (olefins, ethylene hydrocarbons C n H 2n
Homologous series.
|
ethene (ethylene) | |
The simplest alkene is ethylene (C 2 H 4). According to the IUPAC nomenclature, the names of alkenes are formed from the names of the corresponding alkanes by replacing the suffix “-ane” with “-ene”; The position of the double bond is indicated by an Arabic numeral.
Hydrocarbon radicals formed from alkenes have the suffix "-enil". Trivial names: CH 2 =CH- "vinyl", CH 2 =CH-CH 2 - "allyl".
The carbon atoms at the double bond are in a state of sp² hybridization and have a bond angle of 120°.
Alkenes are characterized by isomerism of the carbon skeleton, double bond positions, interclass and spatial.
Physical properties
The melting and boiling points of alkenes (simplified) increase with molecular weight and length of the carbon backbone.
Under normal conditions, alkenes from C 2 H 4 to C 4 H 8 are gases; from pentene C 5 H 10 to hexadecene C 17 H 34 inclusive - liquids, and starting from octadecene C 18 H 36 - solids. Alkenes are insoluble in water, but are highly soluble in organic solvents.
Dehydrogenation of alkanes
This is one of the industrial methods for producing alkenes
Hydrogenation of alkynes
Partial hydrogenation of alkynes requires special conditions and the presence of a catalyst
A double bond is a combination of sigma and pi bonds. A sigma bond occurs when sp2 orbitals overlap axially, and a pi bond occurs when there is lateral overlap.
Zaitsev's rule:
The abstraction of a hydrogen atom in elimination reactions occurs predominantly from the least hydrogenated carbon atom.
13. Alkenes. Structure. sp 2 hybridization, multiple coupling parameters. Reactions of electrophilic addition of halogens, hydrogen halides, hypochlorous acid. Hydration of alkenes. Morkovnikov's rule. Mechanisms of reactions.
Alkenes (olefins, ethylene hydrocarbons) - acyclic unsaturated hydrocarbons, containing one double bond between carbon atoms, forming a homologous series with the general formula C n H 2n
One s-and 2 p-orbitals mix and form 2 equivalent sp2-hybrid orbitals located in the same plane at an angle of 120.
If a bond is formed by more than one pair of electrons, then it is called multiple.
A multiple bond is formed when there are too few electrons and bonding atoms for each bond-forming valence orbital of the central atom to overlap with any orbital of the surrounding atom.
Electrophilic addition reactions
In these reactions, the attacking particle is an electrophile.
Halogenation:
Hydrohalogenation
Electrophilic addition of hydrogen halides to alkenes occurs according to Markovnikov’s rule
Markovnikov rule
Addition of hypochlorous acid to form chlorohydrins:
Hydration
The addition of water to alkenes occurs in the presence of sulfuric acid:
Carbocation- a particle in which a positive charge is concentrated on the carbon atom; the carbon atom has a vacant p-orbital.
14. Ethylene hydrocarbons. Chemical properties: reactions with oxidizing agents. Catalytic oxidation, reaction with peracids, oxidation reaction to glycols, with cleavage of the carbon-carbon bond, ozonation. Wacker process. Substitution reactions.
Alkenes (olefins, ethylene hydrocarbons) - acyclic unsaturated hydrocarbons containing one double bond between carbon atoms, forming a homologous series with the general formula C n H 2n
Oxidation
Oxidation of alkenes can occur, depending on the conditions and types of oxidizing reagents, both with the cleavage of the double bond and with the preservation of the carbon skeleton.
When burned in air, olefins produce carbon dioxide and water.
H 2 C=CH 2 + 3O 2 => 2CO 2 + 2H 2 O
C n H 2n+ 3n/O 2 => nCO 2 + nH 2 O – general formula
Catalytic oxidation
In the presence of palladium salts, ethylene is oxidized to acetaldehyde. Acetone is formed from propene in the same way.
When alkenes are exposed to strong oxidizing agents (KMnO 4 or K 2 Cr 2 O 7 in H 2 SO 4), the double bond breaks when heated:
When alkenes are oxidized with a dilute solution of potassium permanganate, diatomic alcohols are formed - glycols (E.E. Wagner reaction). The reaction takes place in the cold.
Acyclic and cyclic alkenes, when reacting with peracids RCOOOH in a non-polar environment, form epoxides (oxiranes), therefore the reaction itself is called the epoxidation reaction.

Ozonation of alkenes.
When alkenes interact with ozone, peroxide compounds are formed, which are called ozonides. The reaction of alkenes with ozone is the most important method for the oxidative cleavage of alkenes at the double bond
Alkenes do not undergo substitution reactions.
Wacker process-the process of producing acetaldehyde by direct oxidation of ethylene.
The Wacker process is based on the oxidation of ethylene with palladium dichloride:
CH 2 = CH 2 + PdCl 2 + H 2 O = CH 3 CHO + Pd + 2HCl
15. Alkenes: chemical properties. Hydrogenation. Lebedev's rule. Isomerization and oligomerization of alkenes. Radical and ionic polymerization. The concept of polymer, oligomer, monomer, elementary unit, degree of polymerization. Telomerization and copolymerization.
Hydrogenation
Hydrogenation of alkenes directly with hydrogen occurs only in the presence of a catalyst. Hydrogenation catalysts include platinum, palladium, and nickel.
Hydrogenation can also be carried out in the liquid phase with homogeneous catalysts
Isomerization reactions
When heated, isomerization of alkene molecules is possible, which
can lead to both double bond movement and skeletal changes
hydrocarbon.
CH2=CH-CH2-CH3 CH3-CH=CH-CH3
Polymerization reactions
This is a type of addition reaction. Polymerization is the reaction of sequential combination of identical molecules into larger molecules, without isolating any low-molecular-weight product. During polymerization, a hydrogen atom is added to the most hydrogenated carbon atom located at the double bond, and the rest of the molecule is added to the other carbon atom.
CH2=CH2 + CH2=CH2 + ... -CH2-CH2-CH2-CH2- ...
or n CH2=CH2 (-CH2-CH2-)n (polyethylene)
A substance whose molecules undergo a polymerization reaction is called monomer. A monomer molecule must have at least one double bond. The resulting polymers consist of a large number of repeating chains having the same structure ( elementary units). The number showing how many times a structural (elementary) unit is repeated in a polymer is called degree of polymerization(n).
Depending on the type of intermediate particles formed during polymerization, there are 3 polymerization mechanisms: a) radical; b) cationic; c) anionic.
The first method produces high-density polyethylene:
The reaction catalyst is peroxides.
The second and third methods involve the use of acids (cationic polymerization) and organometallic compounds as catalysts.
In chemistry oligomer) - a molecule in the form of a chain of small number of identical constituent links.
Telomerization
Telomerization is the oligomerization of alkenes in the presence of chain transfer agents (telogens). As a result of the reaction, a mixture of oligomers (telomeres) is formed, the end groups of which are parts of telogen. For example, in the reaction of CCl 4 with ethylene, the telogen is CCl 4 .
CCl 4 + nCH 2 =CH 2 => Cl(CH 2 CH 2) n CCl 3
The initiation of these reactions can be carried out by radical initiators or g-radiation.
16. Alkenes. Reactions of radical addition of halogens and hydrogen halides (mechanism). Addition of carbenes to olefins. Ethylene, propylene, butylenes. Industrial sources and main uses.
Alkenes readily add halogens, especially chlorine and bromine (halogenation).
A typical reaction of this type is the discoloration of bromine water
CH2=CH2 + Br2 → CH2Br-CH2Br (1,2-dibromoethane)
The electrophilic addition of hydrogen halides to alkenes occurs according to Markovnikov’s rule:
Markovnikov rule: When adding protic acids or water to unsymmetrical alkenes or alkynemates, hydrogen is added to the most hydrogenated carbon atom
A hydrogenated carbon atom is one that has hydrogen attached to it. Most hydrogenated - where there is most H
Carbene addition reactions
CR 2 carbenes: - highly reactive short-lived species that can easily add to the double bond of alkenes. As a result of the carbene addition reaction, cyclopropane derivatives are formed
Ethylene is an organic chemical described by the formula C 2 H 4. Is the simplest alkene ( olefin)compound. At normal conditions- a colorless flammable gas with a faint odor. Partially soluble in water. Contains a double bond and therefore belongs to unsaturated or unsaturated hydrocarbons. Plays an extremely important role in industry. Ethylene is the most produced organic compound in the world: Ethylene oxide; polyethylene, acetic acid, ethyl alcohol.
Basic chemical properties(don’t teach me, just let them be there just in case, in case they can write it off)
Ethylene is a chemically active substance. Since there is a double bond between the carbon atoms in the molecule, one of them, which is less strong, is easily broken, and at the site of the bond break the attachment, oxidation, and polymerization of molecules occurs.
Halogenation:
CH 2 =CH 2 + Br 2 → CH 2 Br-CH 2 Br
Bromine water becomes discolored. This is a qualitative reaction to unsaturated compounds.
Hydrogenation:
CH 2 =CH 2 + H - H → CH 3 - CH 3 (under the influence of Ni)
Hydrohalogenation:
CH 2 =CH 2 + HBr → CH 3 - CH 2 Br
Hydration:
CH 2 =CH 2 + HOH → CH 3 CH 2 OH (under the influence of a catalyst)
This reaction was discovered by A.M. Butlerov, and it is used for the industrial production of ethyl alcohol.
Oxidation:
Ethylene oxidizes easily. If ethylene is passed through a solution of potassium permanganate, it will become discolored. This reaction is used to distinguish between saturated and unsaturated compounds. Ethylene oxide is a fragile substance; the oxygen bridge breaks and water joins, resulting in the formation of ethylene glycol. Reaction equation:
3CH 2 =CH 2 + 2KMnO 4 + 4H 2 O → 3HOH 2 C - CH 2 OH + 2MnO 2 + 2KOH
C 2 H 4 + 3O 2 → 2CO 2 + 2H 2 O
Polymerization (production of polyethylene):
nCH 2 =CH 2 → (-CH 2 -CH 2 -) n
Propylene(propene) CH 2 = CH-CH 3 - unsaturated (unsaturated) hydrocarbon of the ethylene series, flammable gas. Propylene is a gaseous substance with a low boiling point t boil = −47.6 °C
Typically, propylene is isolated from oil refining gases (during the cracking of crude oil, pyrolysis of gasoline fractions) or associated gases, as well as from coal coking gases.
Unsaturated include hydrocarbons containing multiple bonds between carbon atoms in their molecules. Unlimited are alkenes, alkynes, alkadienes (polyenes). Cyclic hydrocarbons containing a double bond in the ring (cycloalkenes), as well as cycloalkanes with a small number of carbon atoms in the ring (three or four atoms) also have an unsaturated character. The property of “unsaturation” is associated with the ability of these substances to enter into addition reactions, primarily hydrogen, with the formation of saturated, or saturated, hydrocarbons - alkanes.
Structure of alkenes
Acyclic hydrocarbons containing in the molecule, in addition to single bonds, one double bond between carbon atoms and corresponding to the general formula C n H 2n.
Its second name is olefins- alkenes were obtained by analogy with unsaturated fatty acids (oleic, linoleic), the remains of which are part of liquid fats - oils (from the English oil - oil).
Carbon atoms that have a double bond between them are in a state sp 2 -hybridization. This means that one s and two p orbitals are involved in hybridization, and one p orbital remains unhybridized.

The overlap of hybrid orbitals leads to the formation of a σ bond, and due to the unhybridized p orbitals of neighboring carbon atoms, a second, π bond is formed. Thus, a double bond consists of one σ- and one π-bond.
The hybrid orbitals of the atoms forming a double bond are in the same plane, and the orbitals forming a π bond are perpendicular to the plane of the molecule.
The double bond (0.132 nm) is shorter than the single bond, and its energy is higher, because it is stronger. However, the presence of a mobile, easily polarizable π bond leads to the fact that alkenes are chemically more active than alkanes and are able to undergo addition reactions.

Homologous series of alkenes
The first three members of the homologous series of alkenes are gases, from C 5 H 10 to C 17 H 34 are liquids, and from C 18 H 36 are solids. Liquid and solid alkenes are practically insoluble in water, but are highly soluble in organic solvents.
In accordance with IUPAC rules, the suffix -ene is used in the names of homologues of a number of alkenes. The position of the double bond is indicated by a number indicating the location of the bond. The number is placed after the name of the main chain separated by a hyphen. The numbering of atoms in an alkene molecule begins from the end to which the bond is closest, for example, an alkene corresponding to the formula CH 3 −CH 2 −CH=CH−CH 3 should be called penten-2, since the bond begins at the second carbon atom, starting from the end chains.
Unbranched alkenes make up the homologous series of ethene (ethylene): C 2 H 4 - ethene, C 3 H 6 - propene, C 4 H 8 - butene, C 5 H 10 - pentene, C 6 H 12 - hexene, etc.
Isomerism and nomenclature of alkenes
For alkenes, as well as for alkanes, it is characteristic structural isomerism. Structural isomers differ from each other in the structure of the carbon skeleton. The simplest alkene, characterized by structural isomers, is butene.

A special type of structural isomerism is isomerism of the position of the double bond:
Almost free rotation of carbon atoms is possible around a single carbon-carbon bond, so alkane molecules can take on a wide variety of shapes. Rotation around the double bond is impossible, which leads to the appearance of another type of isomerism in alkenes - geometric, or cis-trans isomerism.

Cis-isomers differ from trans-isomers in the spatial arrangement of molecular fragments (in this case, methyl groups) relative to the π-bond plane, and, consequently, in their properties.
Alkenes are isomeric to cycloalkanes (interclass isomerism), for example:

The IUPAC nomenclature for alkenes is similar to that for alkanes.
1. Main circuit selection. The formation of the name of a hydrocarbon begins with the definition of the main chain - the longest chain of carbon atoms in the molecule. In the case of alkenes, the main chain must contain a double bond.
2. Numbering of main chain atoms. The numbering of the atoms of the main chain begins from the end to which the double bond is closest. For example, the correct connection name is:

5-methylhexene-2, not 2-methylhexene-4, as one might expect.
If the position of the double bond cannot determine the beginning of the numbering of atoms in the chain, then it is determined by the position of the substituents in the same way as for saturated hydrocarbons.

3. Formation of the name. The names of alkenes are formed in the same way as the names of alkanes. At the end of the name, indicate the number of the carbon atom at which the double bond begins, and the suffix -ene, indicating that the compound belongs to the class of alkenes. For example:

Physical properties of alkenes
First three representatives of the homologous series of alkenes- gases; substances of the composition C 5 H 10 - C 16 H 32 - liquids; Higher alkenes are solids.
The boiling and melting points naturally increase with increasing molecular weight connections.
Chemical properties of alkenes
Addition reactions. Let us recall that a distinctive feature of representatives of unsaturated hydrocarbons - alkenes is the ability to enter into addition reactions. Most of these reactions proceed according to the mechanism electrophilic addition.
1. Hydrogenation of alkenes. Alkenes are capable of adding hydrogen in the presence of hydrogenation catalysts, metals - platinum, palladium, nickel:
This reaction occurs at atmospheric and elevated pressure and does not require high temperature, because it is exothermic. When the temperature increases, the same catalysts can cause a reverse reaction - dehydrogenation.
2. Halogenation(addition of halogens). The interaction of an alkene with bromine water or a solution of bromine in an organic solvent (CCl 4) leads to rapid discoloration of these solutions as a result of the addition of a halogen molecule to the alkene and the formation of dihaloalkanes:
3. Hydrohalogenation(addition of hydrogen halide).
This reaction obeys Markovnikov's rule:
When a hydrogen halide attaches to an alkene, the hydrogen attaches to the more hydrogenated carbon atom, i.e., the atom at which there are more hydrogen atoms, and the halogen to the less hydrogenated one.
4. Hydration(water connection). Hydration of alkenes leads to the formation of alcohols. For example, the addition of water to ethene underlies one of the industrial methods for producing ethyl alcohol:
Please note that primary alcohol(with a hydroxo group at the primary carbon) is formed only upon hydration of ethene. When propene or other alkenes are hydrated, secondary alcohols are formed.

This reaction also proceeds according to Markovnikov's rule- a hydrogen cation is attached to a more hydrogenated carbon atom, and a hydroxo group is added to a less hydrogenated one.
5. Polymerization. A special case of addition is the polymerization reaction of alkenes:
This addition reaction occurs via a free radical mechanism.
Oxidation reactions.
1. Combustion. Like any organic compounds, alkenes burn in oxygen to form CO 2 and H 2 O:

2. Oxidation in solutions. Unlike alkanes, alkenes are easily oxidized by potassium permanganate solutions. In neutral or alkaline solutions Alkenes are oxidized to diols (dihydric alcohols), and hydroxyl groups are added to those atoms between which a double bond existed before oxidation:

For alkenes, the most typical reactions occur due to the opening of a weaker π bond. In this case, the π bond (in the original alkene) is converted into a σ bond in the reaction product. The original unsaturated compound is converted into a saturated one without the formation of other products, i.e. is happeningaddition reaction.
What is the mechanism of addition reactions to alkenes?
1. Due to the electrons of the π bond, alkene molecules have a region of increased electron density (a cloud of π electrons above and below the plane of the molecule):
Therefore, the double bond is prone to be attacked by an electrophilic (electron-deficient) reagent. In this case, heterolytic cleavage of the π bond will occur and the reaction will proceed along ionic mechanism as electrophilic addition.
The mechanism of electrophilic addition is indicated by the symbol Ad E
(by first letters English terms: Ad – addition [attachment],
E - electrophile [electrophile]).
2. On the other hand, the carbon-carbon π bond, being non-polar, can be broken homolytically, and then the reaction will proceed along radical mechanism.
The radical addition mechanism is indicated by the symbol Ad R
(R – radical - radical).
The mechanism of addition depends on the reaction conditions.
In addition, alkenes are characterized by reactions isomerization And oxidation(including reaction burning, characteristic of all hydrocarbons).
Addition reactions to alkenes
Alkenes undergo a variety of addition reactions.
1. Hydrogenation (addition of hydrogen)
Alkenes react with hydrogen when heated and under elevated pressure in the presence of catalysts (Pt, Pd, Ni, etc.) to form alkanes:
Hydrogenation of alkenes - reverse reaction dehydrogenation of alkanes. According to Le Chatelier's principle, hydrogenation is favored by increased pressure, because this reaction is accompanied by a decrease in the volume of the system.
The addition of hydrogen to carbon atoms in alkenes leads to a decrease in their oxidation state:
Therefore, the hydrogenation of alkenes is classified as a reduction reaction. This reaction is used industrially to produce high-octane fuel.
2. Halogenation (addition of halogens)
The addition of halogens at the C=C double bond occurs easily under normal conditions (at room temperature, without a catalyst). For example, the rapid discoloration of the red-brown color of a solution of bromine in water (bromine water) serves qualitative reaction for the presence of a double bond:
Thus, in the reaction of HCl with propylene, from two possible structural isomers 1-chloropropane and 2-chloropropane, the latter is formed:
This pattern was initially established empirically. In modern organic chemistry, a theoretical justification for Markovnikov’s rule is given based on the position of influence electronic structure molecules on their reactivity.
It should be noted that Markovnikov's rule in its classical formulation is observed only for electrophilic reactions of alkenes themselves. In the case of some alkene derivatives or when the reaction mechanism changes, against the rule Markovnikova.
4. Hydration(water connection)
Hydration occurs in the presence of mineral acids by the mechanism of electrophilic addition:
In reactions of unsymmetrical alkenes, Markovnikov's rule is observed.
1. Polymerization– the reaction of the formation of a high-molecular compound (polymer) by sequential addition of molecules of a low-molecular substance (monomer) according to the scheme:
n M M n
Number n in the polymer formula ( M n ) is called the degree of polymerization. Polymerization reactions of alkenes occur due to addition via multiple bonds:
2. Dimerizationalkenes - the formation of a dimer (double molecule) as a result of an addition reaction. In the presence of a mineral acid (proton donor H + ) a proton is added to the double bond of the alkene molecule. This produces a carbocation:
The "dimeric carbocation" is stabilized by the release of a proton, which leads to the alkene dimerization products - a mixture of isomeric diisobutylenes (2,4,4-trimethylpentene-2 and 2,4,4-trimethylpentene-1):
This process occurs when isobutylene (2-methylpropene) is treated with 60% sulfuric acid at a temperature of 70°C. The resulting mixture of diisobutylenes is hydrogenated to produce "isooctane" (2,2,4-trimethylpentane), which is used to improve the anti-knock ability of gasoline ("isooctane" is a standard 100 octane motor fuel).
Alkenes undergo a variety of reactions in which compounds of other classes are formed. Therefore, alkenes are important intermediates in organic synthesis. When synthesizing many types of substances, it can be useful to first obtain an alkene and then convert it into the desired compound.
All reactions of alkenes can be divided into two groups. One of them is formed by electrophilic addition reactions occurring in two stages, the other by all other reactions. We will begin our consideration below with the second group of reactions.
Hydrogenation
Alkenes react with hydrogen gas in the presence of catalysts (usually noble metals). Two hydrogen atoms are added to the double bond of the alkene to form an alkane. This reaction was discussed in detail in Chap. 3. Let's give two more examples:
Ozonolysis
This reaction is unusual in that it completely breaks the carbon-carbon double bond and splits the carbon skeleton of the molecule into two parts. The alkene is treated with ozone and then with zinc dust. As a result, the alkene molecule splits at the double bond and two molecules of aldehyde and (or) ketone are formed. Acyclic compounds with two aldehyde (or ketone) groups are formed from cycloalkenes:

For example:

Note that in the last two examples, when the cycloalkene ring opens, one acyclic molecule is formed, and not two, as with acyclic alkenes.
The ozonolysis reaction is used both for the synthesis of aldehydes and ketones, and for determining the structure of alkenes. For example, let the ozonolysis of an unknown alkene produce a mixture of two aldehydes:
In this case, the structure of the alkene can be logically established as follows. The carbon atoms linked in aldehyde molecules by double bonds with oxygen atoms were connected by a double bond to each other in the molecule of the original alkene:

Another example:
The alkene structure must be cyclic because we must join two ends of the same molecule:

Oxidation
Diluted water solution Potassium permanganate converts alkenes into diols (glycols). As a result of this reaction, two hydroxyl groups are added to one side of the double bond (cis or syn addition).
Therefore, cis-diols are formed from cycloalkenes. IN general view The reaction equation looks like this:

For example:

The synthesis of diols proceeds best in a slightly alkaline medium and mild conditions (low temperature and a dilute solution of potassium permanganate). Under more severe conditions (acid catalysis, heating), the molecule splits at the double bond and carboxylic acids are formed.
The reaction with potassium permanganate is used not only to produce diols, but also serves as a simple test that allows easy determination of alkenes. The permanganate solution has an intense purple color. If the sample under study contains an alkene, then when a few drops of a permanganate solution are added to it, the violet color of the latter immediately turns brown. Only alkynes and aldehydes cause the same color change. Compounds of most other classes do not react under these conditions. The procedure described above is called the Bayer test. The ratio of compounds of various classes to the Bayer test is shown below: positive sample (purple color disappears), negative sample (purple color remains).
Allyl halogenation
If alkenes are subjected to free radical halogenation, the hydrogen atoms at the carbon atom adjacent to the double bond are most easily replaced by halogen. This position in the alkene molecule is called allylic:

The specific reagent for allylic bromination is -bromosuccinimide. It is a solid substance
which is convenient to work with in the laboratory, while molecular bromine is a volatile, highly toxic and dangerous liquid to handle. When heated (sometimes catalysis by peroxides is necessary), N-bromosuccinimide becomes a source of bromine atoms.

Halogenation proceeds to the allylic position, since the allylic radical formed intermediately is more stable than any other free radical that can be obtained from an alkene molecule. Therefore, it is this radical that is formed more easily than others. The increased stability of the allylic radical is explained by its resonance stabilization, as a result of which the unpaired electron is delocalized over two carbon atoms. The mechanism of allylic chlorination is shown below:

Alkenes are broken down by ozone to form aldehydes and ketones, which allows the structure of alkenes to be determined. Alkenes undergo hydrogenation to form alkanes and oxidation to form diols. In addition to these reactions involving a double bond, alkenes are characterized by selective halogenation to a position adjacent to the double bond. The double bond itself is not affected.
Electrophilic addition to alkenes
Electrophilic addition reactions, differing from each other in the nature of the groups added at the double bond, have the same two-stage mechanism. In its first stage, an electrophilic (having electron affinity) particle (for example, a cation) is attracted by the electron cloud and joins via a double bond.
UNSATURATED, OR UNSATURATED, HYDROCARBONS OF THE ETHYLENE SERIES (ALKENES, OR OLEFINS)
Alkenes, or olefins(from Latin olefiant - oil - an old name, but widely used in chemical literature. The reason for this name was ethylene chloride obtained in XVIII century, - liquid oily substance.) - aliphatic unsaturated hydrocarbons, in the molecules of which there is one double bond between the carbon atoms.
Alkenes form a homologous series with general formula CnH2n
1. Homologous series of alkenes
Homologs:
WITHH2 = CH2 ethene
WITHH2 = CH- CH3 propene
WITHH2=CH-CH2-CH3butene-1
WITHH2=CH-CH2-CH2-CH3 penten-1
Ethylene (ethene) is a colorless gas with a very faint sweetish odor, slightly lighter than air, slightly soluble in water.
C2 - C4 (gases)
C5 - C17 (liquids)
C18 - (solid)
· Alkenes are insoluble in water, soluble in organic solvents (gasoline, benzene, etc.)
Lighter than water
With increasing Mr, the melting and boiling points increase
3. The simplest alkene is ethylene - C2H4
Structural and electronic formula ethylene have the form:
In the ethylene molecule one undergoes hybridization s- and two p-orbitals of C atoms ( sp 2-hybridization).

Thus, each C atom has three hybrid orbitals and one non-hybrid p-orbitals. Two of the hybrid orbitals of the C atoms mutually overlap and form between the C atoms
σ - bond. The remaining four hybrid orbitals of the C atoms overlap in the same plane with four s-orbitals of H atoms and also form four σ - bonds. Two non-hybrid p-orbitals of C atoms mutually overlap in a plane that is located perpendicular to the σ-bond plane, i.e. one is formed P- connection.


By it's nature P- connection is sharply different from σ - connection; P- the bond is less strong due to the overlap of electron clouds outside the plane of the molecule. Under the influence of reagents P- the connection is easily broken.
The ethylene molecule is symmetrical; the nuclei of all atoms are located in the same plane and bond angles are close to 120°; the distance between the centers of C atoms is 0.134 nm.
If atoms are connected by a double bond, then their rotation is impossible without electron clouds P- the connection was not opened.
4. Isomerism of alkenes
Along with structural isomerism of the carbon skeleton Alkenes are characterized, firstly, by other types of structural isomerism - multiple bond position isomerism And interclass isomerism.
Secondly, in the series of alkenes there is spatial isomerism , associated with different positions of substituents relative to the double bond, around which intramolecular rotation is impossible.
Structural isomerism alkenes
1. Isomerism of the carbon skeleton (starting from C4H8):
2. Isomerism of the position of the double bond (starting from C4H8):
3. Interclass isomerism with cycloalkanes, starting with C3H6:
Spatial isomerism of alkenes
Rotation of atoms around a double bond is impossible without breaking it. This is due to the structural features of the p-bond ( p-electron cloud concentrated above and below the plane of the molecule). Due to the rigid fixation of the atoms, rotational isomerism with respect to the double bond does not appear. But it becomes possible cis-trance-isomerism.
Alkenes, which have different substituents on each of the two carbon atoms at the double bond, can exist in the form of two spatial isomers, differing in the location of the substituents relative to the plane of the p-bond. So, in the butene-2 molecule CH3-CH=CH-CH3 CH3 groups can be located either on one side of the double bond in cis-isomer, or on opposite sides in trance-isomer.

ATTENTION! cis-trans- Isomerism does not appear if at least one of the C atoms at the double bond has 2 identical substituents.
For example,
butene-1 CH2=CH-CH2-CH3 doesn't have cis- And trance-isomers, because The 1st C atom is bonded to two identical H atoms.
Isomers cis- And trance- differ not only physically
 ,
,
but also chemical properties, because bringing parts of a molecule closer or further away from each other in space promotes or hinders chemical interaction.
Sometimes cis-trans-isomerism is not quite accurately called geometric isomerism. The inaccuracy is that All spatial isomers differ in their geometry, and not only cis- And trance-.
5. Nomenclature
Alkenes simple structure often called by replacing the suffix -ane in alkanes with -ylene: ethane - ethylene, propane - propylene, etc.
By systematic nomenclature The names of ethylene hydrocarbons are made by replacing the suffix -ane in the corresponding alkanes with the suffix -ene (alkane - alkene, ethane - ethene, propane - propene, etc.). The choice of the main chain and the naming order are the same as for alkanes. However, the chain must necessarily include a double bond. The numbering of the chain begins from the end to which this connection is located closest. For example:

Unsaturated (alkene) radicals are called by trivial names or by systematic nomenclature:
(H2C=CH—) vinyl or ethenyl
(H2C=CH—CH2) allyl