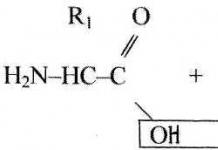
(ORGANIC CHEMISTRY)
Splitting of water from alcohols (dehydration):
Acid reagents are used as dehydration catalysts: sulfuric and phosphoric acids, alumina, etc. The order of splitting off is most often determined by Zaitsev's rule (1875): during the formation of water, hydrogen is most easily split off from the neighboring least hydrogenated carbon atom...(ORGANIC CHEMISTRY)
Alcohol oxidation
Alcohols are more easily oxidized than hydrocarbons, and the carbon at which the hydroxyl group is located is the first to be oxidized. The most suitable oxidizing agent in laboratory conditions is a chromium mixture. In industry - atmospheric oxygen in the presence of catalysts. Primary...(ORGANIC CHEMISTRY)
Oxidation of ethyl alcohol to acetic acid.
Ethyl alcohol is oxidized to acetic acid under the influence of acetic acid bacteria of the genera Gluconobacter and Acetobacter. They are Gram-negative chemoorganoheterotrophic, non-spore-forming, rod-shaped organisms, motile or immobile. Acetic acid bacteria of these genera differ from each other in ...(FUNDAMENTALS OF MICROBIOLOGY)
Catalytic dehydrogenation of paraffins
An important industrial method is also the catalytic dehydrogenation of paraffins over chromium oxide: Most laboratory methods for obtaining olefins are based on the reactions of elimination (elimination) of various reagents: water, halogens or hydrogen halides from the corresponding derivatives of saturated ...(ORGANIC CHEMISTRY)
The principal problem that arises in the oxidation of alcohols to aldehydes is that aldehydes are very easily subjected to further oxidation compared to the parent alcohols. In fact, aldehydes are active organic reducing agents. So, when oxidized primary alcohols sodium bichromate in sulfuric acid (Beckmann mixture), the aldehyde that is formed must be protected from further oxidation to a carboxylic acid. It is possible, for example, to remove the aldehyde from the reaction mixture. And this is widely used, since the boiling point of the aldehyde is usually lower than the boiling point of the original alcohol. In this way, first of all, low-boiling aldehydes can be obtained, for example, acetic, propionic, isobutyric:
Picture 1.
Better results can be obtained if glacial acetic acid is used instead of sulfuric acid.
To obtain high-boiling aldehydes from the corresponding primary alcohols, chromic acid tert-butyl ester is used as an oxidizing agent:
Figure 2.
In the oxidation of unsaturated alcohols with tert-butyl chromate (in aprotic nonpolar solvents), multiple bonds are not engaged, and unsaturated aldehydes are formed in high yields.
Sufficiently selective is the oxidation method, which uses manganese dioxide in an organic solvent, pentane or methylene chloride. For example, allyl and benzyl alcohols can thus be oxidized to the corresponding aldehydes. Output alcohols are slightly soluble in non-polar solvents, and aldehydes, which are formed as a result of oxidation, are much better soluble in pentane or methylene chloride. Therefore, carbonyl compounds pass into the solvent layer and thus contact with the oxidizing agent and further oxidation can be prevented:
Figure 3
It is much easier to oxidize secondary alcohols to ketones than to oxidize primary alcohols to aldehydes. The yields are higher here because, firstly, reactivity secondary alcohols is higher than primary, and, secondly, ketones, which are formed much more resistant to oxidizing agents than aldehydes.
Oxidizing agents for the oxidation of alcohols
For the oxidation of alcohols as oxidizing agents, reagents based on transition metals- derivatives of hexavalent chromium, four and seven valent manganese.
For the selective oxidation of primary alcohols to aldehydes, the $CrO_3$ complex with pyridine - $CrO_(3^.) 2C_5H_5N$ (Sarrett-Collins reagent) is currently considered to be the best reagent. +H$ in methylene chloride. The red $CrO_(3^.) 2C_5H_5N$ complex is obtained by slow interaction of $CrO_(3^.)$ with pyridine at 10-15 $^\circ$C. Orange pyridinium chlorochromate is obtained by adding pyridine to a solution of chromium (IV) oxide in 20% hydrochloric acid. Both of these reagents are soluble in $CH_2Cl_2$ or $CHCl_3$:
Figure 4
These reagents provide very high yields of aldehydes, but pyridinium chlorochromate has the important advantage that this reagent does not affect the double or triple bonds in the starting alcohols and is therefore particularly effective for the preparation of unsaturated aldehydes.
To obtain $α¸β$-unsaturated aldehydes by oxidation of substituted allyl alcohols, manganese(IV) oxide $MnO_2$
Examples of reactions of alcohols with these oxidizing agents are given below:
Catalytic dehydrogenation of alcohols
Strictly speaking, the oxidation of alcohols to carbonyl compounds is reduced to the elimination of hydrogen from the molecule of the original alcohol. Such cleavage can be carried out not only using the previously discussed oxidation methods, but also using catalytic dehydrogenation. Catalytic dehydrogenation is the process of splitting hydrogen from alcohols in the presence of a catalyst (copper, silver, zinc oxide, a mixture of chromium and copper oxides) both with and without oxygen. The dehydrogenation reaction in the presence of oxygen is called the oxidative dehydrogenation reaction.
Finely dispersed copper and silver, as well as zinc oxide, are most often used as catalysts. The catalytic dehydrogenation of alcohols is especially convenient for the synthesis of aldehydes, which are very easily oxidized to acids.
The above catalysts are applied in a highly dispersed state on inert carriers with a developed surface, for example, asbestos, pumice. The equilibrium of the catalytic dehydrogenation reaction is established at a temperature of 300-400 $^\circ$C. To prevent further transformation of the dehydrogenation products, the reaction gases must be rapidly cooled. Dehydrogenation is a very endothermic reaction ($\triangle H$ = 70-86 kJ/mol). Hydrogen formed can be burned if air is added to the reaction mixture, then the overall reaction will be highly exothermic ($\triangle H$ = -(160-180) kJ / mol). This process is called oxidative dehydrogenation or autothermal dehydrogenation. Although dehydrogenation is used mainly in industry, this method can also be used in the laboratory for preparative synthesis.
Saturation dehydrogenation of aliphatic alcohols occurs in good yields:
Figure 9
In the case of high-boiling alcohols, the reaction is carried out under reduced pressure. Unsaturated alcohols under dehydrogenation conditions are converted into the corresponding saturated carbonyl compounds. Hydrogenation of the multiple $C = C$ bond occurs with hydrogen, which is formed during the reaction. To prevent this side reaction and to be able to obtain unsaturated carbonyl compounds by catalytic dehydrogenation, the process is carried out in a vacuum at 5-20 mm Hg. Art. in the presence of water vapor. This method allows you to get a number of unsaturated carbonyl compounds:
Figure 10.
Application of alcohol dehydrogenation
The dehydrogenation of alcohols is an important industrial method for the synthesis of aldehydes and ketones, such as formaldehyde, acetaldehyde, and acetone. These products are produced in large volumes by both dehydrogenation and oxidative dehydrogenation on a copper or silver catalyst.
Depending on the type of hydrocarbon radical, and also, in some cases, the features of attaching the -OH group to this hydrocarbon radical of a compound with a hydroxyl functional group divided into alcohols and phenols.
alcohols refers to compounds in which the hydroxyl group is attached to the hydrocarbon radical, but is not attached directly to the aromatic nucleus, if any, in the structure of the radical.
Examples of alcohols:
If the structure of the hydrocarbon radical contains an aromatic nucleus and a hydroxyl group, and is connected directly to the aromatic nucleus, such compounds are called phenols .
Examples of phenols:
Why are phenols classified in a separate class from alcohols? After all, for example, formulas

very similar and give the impression of substances of the same class organic compounds.
However, the direct connection of the hydroxyl group with the aromatic nucleus significantly affects the properties of the compound, since the conjugated system of π-bonds of the aromatic nucleus is also conjugated with one of the lone electron pairs of the oxygen atom. Because of this, the O-H bond in phenols is more polar than in alcohols, which significantly increases the mobility of the hydrogen atom in the hydroxyl group. In other words, phenols are much brighter than alcohols. acid properties.
Chemical properties of alcohols
Monohydric alcohols
Substitution reactions
Substitution of a hydrogen atom in the hydroxyl group
1) Alcohols react with alkali, alkaline earth metals and aluminum (purified from the protective film of Al 2 O 3), while metal alcoholates are formed and hydrogen is released:
The formation of alcoholates is possible only when using alcohols that do not contain water dissolved in them, since alcoholates are easily hydrolyzed in the presence of water:
CH 3 OK + H 2 O \u003d CH 3 OH + KOH
2) Esterification reaction
The esterification reaction is the interaction of alcohols with organic and oxygen-containing inorganic acids, leading to the formation of esters.
This type of reaction is reversible, therefore, in order to shift the equilibrium towards the formation of an ester, it is desirable to carry out the reaction under heating, as well as in the presence of concentrated sulfuric acid as a water-removing agent:
Substitution of the hydroxyl group
1) When alcohols are treated with halogen acids, the hydroxyl group is replaced by a halogen atom. As a result of this reaction, haloalkanes and water are formed:
2) By passing a mixture of alcohol vapors with ammonia through heated oxides of some metals (most often Al 2 O 3), primary, secondary or tertiary amines can be obtained:
The type of amine (primary, secondary, tertiary) will depend to some extent on the ratio of the starting alcohol and ammonia.
Elimination reactions (cleavage)
Dehydration
Dehydration, which actually involves the splitting off of water molecules, in the case of alcohols differs by intermolecular dehydration and intramolecular dehydration.
At intermolecular dehydration alcohols, one water molecule is formed as a result of the elimination of a hydrogen atom from one alcohol molecule and a hydroxyl group from another molecule.
As a result of this reaction, compounds belonging to the class of ethers (R-O-R) are formed:
intramolecular dehydration alcohols proceeds in such a way that one molecule of water is split off from one molecule of alcohol. This type of dehydration requires somewhat more stringent conditions, consisting in the need to use a markedly higher heating compared to intermolecular dehydration. In this case, one alkene molecule and one water molecule are formed from one alcohol molecule:
Since the methanol molecule contains only one carbon atom, intramolecular dehydration is impossible for it. During the dehydration of methanol, only ether(CH 3 -O-CH 3).
It is necessary to clearly understand the fact that in the case of dehydration of unsymmetrical alcohols, intramolecular elimination of water will proceed in accordance with the Zaitsev rule, i.e. hydrogen will be split off from the least hydrogenated carbon atom:
Dehydrogenation of alcohols
a) Dehydrogenation of primary alcohols when heated in the presence of metallic copper leads to the formation aldehydes:
b) In the case of secondary alcohols, similar conditions will lead to the formation ketones:
c) Tertiary alcohols do not enter into a similar reaction, i.e. are not dehydrated.
Oxidation reactions
Combustion
Alcohols readily react with combustion. This produces a large amount of heat:
2CH 3 -OH + 3O 2 \u003d 2CO 2 + 4H 2 O + Q
incomplete oxidation
Incomplete oxidation of primary alcohols can lead to the formation of aldehydes and carboxylic acids.
In the case of incomplete oxidation of secondary alcohols, the formation of only ketones is possible.
Incomplete oxidation of alcohols is possible when they are exposed to various oxidizing agents, such as air oxygen in the presence of catalysts (copper metal), potassium permanganate, potassium dichromate, etc.
In this case, aldehydes can be obtained from primary alcohols. As you can see, the oxidation of alcohols to aldehydes, in fact, leads to the same organic products as dehydrogenation:
It should be noted that when using oxidizing agents such as potassium permanganate and potassium dichromate in acidic environment a deeper oxidation of alcohols, namely to carboxylic acids, is possible. In particular, this manifests itself when using an excess of an oxidizing agent during heating. Secondary alcohols can only oxidize to ketones under these conditions.
LIMITED POLYTOMIC ALCOHOLS
Substitution of hydrogen atoms of hydroxyl groups
Polyhydric alcohols as well as monohydric react with alkali, alkaline earth metals and aluminum (cleaned from the filmAl 2 O 3 ); in this case, a different number of hydrogen atoms of hydroxyl groups in an alcohol molecule can be replaced:
2. Since the molecules of polyhydric alcohols contain several hydroxyl groups, they influence each other due to the negative inductive effect. In particular, this leads to a weakening O-N connections and increase the acidic properties of hydroxyl groups.
B about The greater acidity of polyhydric alcohols is manifested in the fact that polyhydric alcohols, in contrast to monohydric ones, react with some hydroxides of heavy metals. For example, you need to remember the fact that freshly precipitated copper hydroxide reacts with polyhydric alcohols to form a bright blue solution. complex compound.
Thus, the interaction of glycerol with freshly precipitated copper hydroxide leads to the formation of a bright blue solution of copper glycerate:
This reaction is qualitative for polyhydric alcohols. For passing the exam it is enough to know the signs of this reaction, and it is not necessary to be able to write the interaction equation itself.
3. Just like monohydric alcohols, polyhydric ones can enter into an esterification reaction, i.e. react with organic and oxygen-containing inorganic acids to form esters. This reaction is catalyzed by strong inorganic acids and is reversible. In this regard, during the esterification reaction, the resulting ester is distilled off from the reaction mixture in order to shift the equilibrium to the right according to the Le Chatelier principle:
If they react with glycerin carboxylic acids with a large number of carbon atoms in the hydrocarbon radical resulting from such a reaction, esters are called fats.
In the case of esterification of alcohols with nitric acid, the so-called nitrating mixture is used, which is a mixture of concentrated nitric and sulfuric acids. The reaction is carried out under constant cooling:
Ester glycerin and nitric acid, called trinitroglycerin, is an explosive. In addition, a 1% solution of this substance in alcohol has a powerful vasodilating effect, which is used for medical indications to prevent a stroke or heart attack.
Substitution of hydroxyl groups
Reactions of this type proceed according to the mechanism nucleophilic substitution. Interactions of this kind include the reaction of glycols with hydrogen halides.
So, for example, the reaction of ethylene glycol with hydrogen bromide proceeds with the successive replacement of hydroxyl groups by halogen atoms:
Chemical properties of phenols
As mentioned at the very beginning of this chapter, the chemical properties of phenols differ markedly from those of alcohols. This is due to the fact that one of the lone electron pairs of the oxygen atom in the hydroxyl group is conjugated with the π-system of conjugated bonds of the aromatic ring.
Reactions involving the hydroxyl group
Acid properties
Phenols are more strong acids than alcohols, and in aqueous solution are dissociated to a very small extent:
B about The greater acidity of phenols compared to alcohols in terms of chemical properties is expressed in the fact that phenols, unlike alcohols, are able to react with alkalis:
However, the acidic properties of phenol are less pronounced than even one of the weakest inorganic acids - carbonic. So, in particular, carbon dioxide, when passing it through water solution alkali metal phenolates, displaces free phenol from the latter as an acid even weaker than carbonic acid:
Obviously, any other stronger acid will also displace phenol from phenolates:
3) Phenols are stronger acids than alcohols, while alcohols react with alkali and alkaline earth metals. In this regard, it is obvious that phenols will also react with these metals. The only thing is that, unlike alcohols, the reaction of phenols with active metals requires heating, since both phenols and metals are solids:
Substitution reactions in the aromatic nucleus
The hydroxyl group is a substituent of the first kind, which means that it facilitates substitution reactions in ortho- and pair- positions in relation to oneself. Reactions with phenol proceed under much milder conditions than with benzene.
Halogenation
The reaction with bromine does not require any special conditions. When bromine water is mixed with a solution of phenol, a white precipitate of 2,4,6-tribromophenol is instantly formed:
Nitration
When a mixture of concentrated nitric and sulfuric acids (nitrating mixture) acts on phenol, 2,4,6-trinitrophenol is formed - a yellow crystalline explosive:
Addition reactions
Since phenols are unsaturated compounds, they can be hydrogenated in the presence of catalysts to the corresponding alcohols.
The generally accepted mechanism for the dehydration of alcohols is as follows (for simplicity, ethyl alcohol is taken as an example):
The alcohol adds a hydrogen ion in step (1) to form a protonated alcohol, which dissociates in step (2) to give a water molecule and a carbonium ion; then the carbonium ion in step (3) loses a hydrogen ion and an alkene is formed.
Thus, the double bond is formed in two steps: loss of the hydroxyl group as [step (2)] and loss of hydrogen (step (3)). This is the difference between this reaction and the dehydrohalogenation reaction, where the elimination of hydrogen and halogen occurs simultaneously.
The first stage represents the Brønsted-Lowry acid-base balance (section 1.19). When sulfuric acid is dissolved in water, for example, the following reaction occurs:

The hydrogen ion went from a very weak base to a stronger base to form the oxonium ion. The main properties of both compounds are, of course, due to the lone pair of electrons that can bind the hydrogen ion. Alcohol also contains an oxygen atom with a lone pair of electrons and its basicity is comparable to that of water. The first stage of the proposed mechanism can most likely be represented as follows:

The hydrogen ion has moved from the bisulfate ion to a stronger base ( ethyl alcohol) to form a substituted oxonium ion of the protonated alcohol.
Similarly, step (3) is not the expulsion of a free hydrogen ion, but its transition to the strongest base available, namely to

For convenience, this process is often depicted as the addition or elimination of a hydrogen ion, but it should be understood that in all cases, in fact, there is a transfer of a proton from one base to another.
All three reactions are given as equilibrium because each step is reversible; as will be shown below, the reverse reaction is the formation of alcohols from alkenes (Sec. 6.10). Equilibrium (1) is shifted very strongly to the right; it is known that sulfuric acid almost completely ionized in alcoholic solution. Since the concentration of carbonium ions available at any moment is very small, equilibrium (2) is shifted strongly to the left. At some point, one of these few carbonium ions reacts according to equation (3) to form an alkene. During dehydration, the volatile alkene is usually distilled off from the reaction mixture, and thus the equilibrium (3) shifts to the right. As a result, the entire reaction comes to an end.
The carbonium ion is formed as a result of the dissociation of the protonated alcohol; the charged particle is separated from
neutral particle Obviously, this process requires much less energy than the formation of a carbonium ion from the alcohol itself, since in this case it is necessary to separate the positive particle from the negative one. In the first case, a weak base (water) is split off from a carbonium ion (Lewis acid) much more easily than a very strong base, the hydroxyl ion, i.e. water is a better leaving group than the hydroxyl ion. It has been shown that the hydroxyl ion almost never splits off from alcohol; bond cleavage reactions in alcohol in almost all cases require an acid catalyst, the role of which, as in the present case, is to protonate the alcohol.

Finally, it should be understood that the dissociation of the protonated alcohol becomes possible only due to the solvation of the carbonium ion (cf. Section 5.14). The energy to break the carbon-oxygen bond is taken from the formation a large number ion-dipole bonds between a carbonium ion and a polar solvent.
The carbonium ion can enter into various reactions; which one occurs depends on the experimental conditions. All reactions of carbonium ions end in the same way: they acquire a pair of electrons to fill an octet at a positively charged carbon atom. In this case, a hydrogen ion is split off from a carbon atom adjacent to a positively charged, electron-depleted carbon atom; a pair of electrons that previously bonded with this hydrogen can now form a -bond

This mechanism explains acid catalysis during dehydration. Does this mechanism also explain the fact that the ease of dehydration of alcohols decreases in the series tertiary secondary primary? Before answering this question, it is necessary to find out how the stability of carbonium ions changes.
Alcohol dehydrogenation reactions are necessary to produce aldehydes and ketones. Ketones are obtained from secondary alcohols, and aldehydes from primary alcohols. Copper, silver, copper chromites, zinc oxide, etc. serve as catalysts in the processes. It should be noted that, compared to copper catalysts, zinc oxide is more stable and does not lose activity during the process, however, it can provoke a dehydration reaction. AT general view alcohol dehydrogenation reactions can be represented as follows:
In industry, the dehydrogenation of alcohols produces compounds such as acetaldehyde, acetone, methyl ethyl ketone, and cyclohexanone. The processes proceed in a stream of water vapor. The most common processes are:
1. carried out on a copper or silver catalyst at a temperature of 200 - 400 ° C and atmospheric pressure. The catalyst is some kind of Al 2 O 3 , SnO 2 or carbon fiber supported with silver or copper components. This reaction is one of the components of the Wacker process, which is an industrial method for obtaining acetaldehyde from ethanol by dehydrogenation or oxidation with oxygen.

2. may proceed differently, depending on structural formula its original substance. 2-propanol, which is a secondary alcohol, is dehydrogenated to acetone, and 1-propanol, being a primary alcohol, is dehydrogenated to propanal at atmospheric pressure and a process temperature of 250–450 °C.


3. also depends on the structure of the starting compound, which affects the final product (aldehyde or ketone).

4. Methanol dehydrogenation. This process is not fully understood, but most researchers highlight it as a promising process for the synthesis of formaldehyde that does not contain water. Various process parameters are proposed: temperature 600 - 900 °C, active component of the catalyst zinc or copper, silicon oxide carrier, the possibility of initiating the reaction with hydrogen peroxide, etc. At the moment, most of the formaldehyde in the world is produced by the oxidation of methanol.