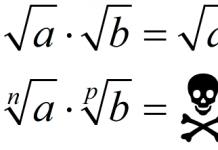The atmosphere is the gaseous shell of our planet, which rotates along with the Earth. The gas in the atmosphere is called air. The atmosphere is in contact with the hydrosphere and partially covers the lithosphere. But the upper limits are difficult to determine. It is conventionally accepted that the atmosphere extends upward for approximately three thousand kilometers. There it smoothly flows into airless space.
Chemical composition of the Earth's atmosphere
The formation of the chemical composition of the atmosphere began about four billion years ago. Initially, the atmosphere consisted only of light gases - helium and hydrogen. According to scientists, the initial prerequisites for the creation of a gas shell around the Earth were volcanic eruptions, which, along with lava, emitted huge amounts of gases. Subsequently, gas exchange began with water spaces, with living organisms, and with the products of their activities. The composition of the air gradually changed and was fixed in its modern form several million years ago.

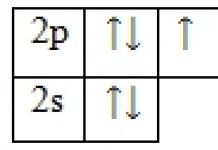
The main components of the atmosphere are nitrogen (about 79%) and oxygen (20%). The remaining percentage (1%) is made up of the following gases: argon, neon, helium, methane, carbon dioxide, hydrogen, krypton, xenon, ozone, ammonia, sulfur and nitrogen dioxides, nitrous oxide and carbon monoxide, which are included in this one percent.
In addition, the air contains water vapor and particulate matter (pollen, dust, salt crystals, aerosol impurities).
Recently, scientists have noted not a qualitative, but a quantitative change in some air ingredients. And the reason for this is man and his activities. In the last 100 years alone, carbon dioxide levels have increased significantly! This is fraught with many problems, the most global of which is climate change.
Formation of weather and climate
The atmosphere plays a critical role in shaping the climate and weather on Earth. A lot depends on the amount of sunlight, the nature of the underlying surface and atmospheric circulation.

Let's look at the factors in order.
1. The atmosphere transmits the heat of the sun's rays and absorbs harmful radiation. The ancient Greeks knew that the rays of the Sun fall on different parts of the Earth at different angles. The word “climate” itself translated from ancient Greek means “slope”. So, at the equator, the sun's rays fall almost vertically, which is why it is very hot here. The closer to the poles, the greater the angle of inclination. And the temperature drops.
2. Due to the uneven heating of the Earth, air currents are formed in the atmosphere. They are classified according to their sizes. The smallest (tens and hundreds of meters) are local winds. This is followed by monsoons and trade winds, cyclones and anticyclones, and planetary frontal zones.
All these air masses are constantly moving. Some of them are quite static. For example, trade winds that blow from the subtropics towards the equator. The movement of others depends largely on atmospheric pressure.
3. Atmospheric pressure is another factor influencing climate formation. This is the air pressure on the surface of the earth. As is known, air masses move from an area with high atmospheric pressure towards an area where this pressure is lower.
A total of 7 zones are allocated. The equator is a low pressure zone. Further, on both sides of the equator up to the thirties latitudes there is an area of high pressure. From 30° to 60° - low pressure again. And from 60° to the poles is a high pressure zone. Air masses circulate between these zones. Those that come from the sea to land bring rain and bad weather, and those that blow from the continents bring clear and dry weather. In places where air currents collide, atmospheric front zones are formed, which are characterized by precipitation and inclement, windy weather.
Scientists have proven that even a person’s well-being depends on atmospheric pressure. According to international standards, normal atmospheric pressure is 760 mm Hg. column at a temperature of 0°C. This indicator is calculated for those areas of land that are almost level with sea level. With altitude the pressure decreases. Therefore, for example, for St. Petersburg 760 mm Hg. - this is the norm. But for Moscow, which is located higher, normal pressure is 748 mm Hg.
The pressure changes not only vertically, but also horizontally. This is especially felt during the passage of cyclones.
The structure of the atmosphere
The atmosphere is reminiscent of a layer cake. And each layer has its own characteristics.

. Troposphere- the layer closest to the Earth. The "thickness" of this layer changes with distance from the equator. Above the equator, the layer extends upward by 16-18 km, in temperate zones by 10-12 km, at the poles by 8-10 km.
It is here that 80% of the total air mass and 90% of water vapor are contained. Clouds form here, cyclones and anticyclones arise. The air temperature depends on the altitude of the area. On average, it decreases by 0.65° C for every 100 meters.
. Tropopause- transition layer of the atmosphere. Its height ranges from several hundred meters to 1-2 km. The air temperature in summer is higher than in winter. For example, above the poles in winter it is -65° C. And above the equator it is -70° C at any time of the year.
. Stratosphere- this is a layer whose upper boundary lies at an altitude of 50-55 kilometers. Turbulence here is low, the content of water vapor in the air is negligible. But there is a lot of ozone. Its maximum concentration is at an altitude of 20-25 km. In the stratosphere, the air temperature begins to rise and reaches +0.8° C. This is due to the fact that the ozone layer interacts with ultraviolet radiation.
. Stratopause- a low intermediate layer between the stratosphere and the mesosphere that follows it.
. Mesosphere- the upper boundary of this layer is 80-85 kilometers. Complex photochemical processes involving free radicals occur here. They are the ones who provide that gentle blue glow of our planet, which is seen from space.
Most comets and meteorites burn up in the mesosphere.
. Mesopause- the next intermediate layer, the air temperature in which is at least -90°.
. Thermosphere- the lower boundary begins at an altitude of 80 - 90 km, and the upper boundary of the layer runs approximately at 800 km. The air temperature is rising. It can vary from +500° C to +1000° C. During the day, temperature fluctuations amount to hundreds of degrees! But the air here is so rarefied that understanding the term “temperature” as we imagine it is not appropriate here.
. Ionosphere- combines the mesosphere, mesopause and thermosphere. The air here consists mainly of oxygen and nitrogen molecules, as well as quasi-neutral plasma. The sun's rays entering the ionosphere strongly ionize air molecules. In the lower layer (up to 90 km) the degree of ionization is low. The higher, the greater the ionization. So, at an altitude of 100-110 km, electrons are concentrated. This helps to reflect short and medium radio waves.
The most important layer of the ionosphere is the upper one, which is located at an altitude of 150-400 km. Its peculiarity is that it reflects radio waves, and this facilitates the transmission of radio signals over considerable distances.

It is in the ionosphere that such a phenomenon as the aurora occurs.
. Exosphere- consists of oxygen, helium and hydrogen atoms. The gas in this layer is very rarefied and hydrogen atoms often escape into outer space. Therefore, this layer is called the “dispersion zone”.
The first scientist to suggest that our atmosphere has weight was the Italian E. Torricelli. Ostap Bender, for example, in his novel “The Golden Calf” lamented that every person is pressed by a column of air weighing 14 kg! But the great schemer was a little mistaken. An adult experiences pressure of 13-15 tons! But we do not feel this heaviness, because atmospheric pressure is balanced by the internal pressure of a person. The weight of our atmosphere is 5,300,000,000,000,000 tons. The figure is colossal, although it is only a millionth of the weight of our planet.
The Earth's atmosphere is the gaseous envelope of our planet. By the way, almost all celestial bodies have similar shells, from the planets of the solar system to large asteroids. depends on many factors - the size of its speed, mass and many other parameters. But only the shell of our planet contains the components that allow us to live.
Earth's atmosphere: a brief history of its origin
It is believed that at the beginning of its existence, our planet did not have a gaseous shell at all. But the young, newly formed celestial body was constantly evolving. The Earth's primary atmosphere was formed as a result of constant volcanic eruptions. This is how, over many thousands of years, a shell of water vapor, nitrogen, carbon and other elements (except oxygen) formed around the Earth.
Since the amount of moisture in the atmosphere is limited, its excess turned into precipitation - this is how seas, oceans and other bodies of water were formed. The first organisms that populated the planet appeared and developed in the aquatic environment. Most of them belonged to plant organisms that produce oxygen through photosynthesis. Thus, the Earth's atmosphere began to fill with this vital gas. And as a result of the accumulation of oxygen, the ozone layer was formed, which protected the planet from the harmful effects of ultraviolet radiation. It is these factors that created all the conditions for our existence.
The structure of the Earth's atmosphere
As you know, the gas shell of our planet consists of several layers - the troposphere, stratosphere, mesosphere, thermosphere. It is impossible to draw clear boundaries between these layers - it all depends on the time of year and the latitude of the planet.
The troposphere is the lower part of the gas shell, the height of which averages from 10 to 15 kilometers. This is where most of the moisture is concentrated. By the way, this is where all the moisture is located and clouds form. Due to the oxygen content, the troposphere supports the life activity of all organisms. In addition, it is crucial in shaping the weather and climatic features of the area - not only clouds, but also winds are formed here. Temperature drops with altitude.
Stratosphere - starts from the troposphere and ends at an altitude of 50 to 55 kilometers. Here the temperature increases with altitude. This part of the atmosphere contains virtually no water vapor, but does have an ozone layer. Sometimes here you can notice the formation of “pearl” clouds, which can only be seen at night - they are believed to be represented by highly condensed water drops.
The mesosphere stretches up to 80 kilometers up. In this layer you can notice a sharp drop in temperature as you move up. Turbulence is also highly developed here. By the way, so-called “noctilucent clouds” are formed in the mesosphere, which consist of small ice crystals - they can only be seen at night. It is interesting that there is practically no air at the upper boundary of the mesosphere - it is 200 times less than near the earth's surface.
The thermosphere is the upper layer of the earth's gas shell, in which it is customary to distinguish between the ionosphere and the exosphere. Interestingly, the temperature here rises very sharply with altitude - at an altitude of 800 kilometers from the earth's surface it is more than 1000 degrees Celsius. The ionosphere is characterized by highly thin air and a huge content of active ions. As for the exosphere, this part of the atmosphere smoothly passes into interplanetary space. It is worth noting that the thermosphere does not contain air.
It can be noted that the Earth's atmosphere is a very important part of our planet, which remains a decisive factor in the emergence of life. It ensures life activity, maintains the existence of the hydrosphere (the watery shell of the planet) and protects from ultraviolet radiation.
Page 4 of 10
The formation of the Earth's atmosphere began in ancient times - during the protoplanetary stage of the Earth's development, during a period of active volcanic eruptions with the release of huge amounts of gases. Later, when oceans and the biosphere appeared on Earth, the formation of the atmosphere continued due to gas exchange between water, plants, animals and the products of their decomposition.
Throughout geological history, the Earth's atmosphere has undergone a number of profound transformations.
 The Earth's primary atmosphere. Restorative.
The Earth's primary atmosphere. Restorative.
Part Earth's primary atmosphere at the protoplanetary stage of the Earth's development (more than 4.2 billion years ago) it consisted mainly of methane, ammonia and carbon dioxide. Then, as a result of degassing of the Earth's mantle and continuous weathering processes on the earth's surface, the composition of the Earth's primary atmosphere was enriched with water vapor, carbon (CO 2 , CO) and sulfur compounds, as well as strong halogen acids (HCI, HF, HI) and boric acid. The primary atmosphere was very thin.
Secondary atmosphere of the Earth. Oxidative.
Subsequently, the primary atmosphere began to transform into a secondary one. This happened as a result of the same weathering processes that occurred on the surface of the earth, volcanic and solar activity, as well as due to the activity of cyanobacteria and blue-green algae.
The result of the transformation was the decomposition of methane into hydrogen and carbon dioxide, and ammonia into nitrogen and hydrogen. Carbon dioxide and nitrogen began to accumulate in the Earth's atmosphere.
Blue-green algae began to produce oxygen through photosynthesis, which was almost all spent on the oxidation of other gases and rocks. As a result, ammonia was oxidized to molecular nitrogen, methane and carbon monoxide to carbon dioxide, sulfur and hydrogen sulfide to SO 2 and SO 3.
Thus, the atmosphere gradually turned from reducing to oxidizing.
Formation and evolution of carbon dioxide in the primary and secondary atmosphere.
Sources of carbon dioxide in the early stages of the formation of the Earth's atmosphere:
- Methane oxidation,
- Degassing of the Earth's mantle,
- Weathering of rocks.
At the turn of the Proterozoic and Paleozoic (ca. 600 million years ago), the content of carbon dioxide in the atmosphere decreased and amounted to only tenths of a percent of the total volume of gases in the atmosphere.
Carbon dioxide reached its current level in the atmosphere only 10-20 million years ago.
Formation and evolution of oxygen in the primary and secondary atmosphere of the Earth.
Oxygen sources in the early stages of atmospheric formation Lands:
- Degassing of the Earth's mantle - almost all the oxygen was spent on oxidative processes.
- Photodissociation of water (decomposition into hydrogen and oxygen molecules) in the atmosphere under the influence of ultraviolet radiation - as a result, free oxygen molecules appeared in the atmosphere.
- Conversion of carbon dioxide into oxygen by eukaryotes. The appearance of free oxygen in the atmosphere led to the death of prokaryotes (adapted to living in reducing conditions) and the emergence of eukaryotes (adapted to living in an oxidizing environment).
Changes in oxygen concentration in the Earth's atmosphere.
Archean - first half of the Proterozoic – oxygen concentration is 0.01% of the modern level (Yuri point). Almost all of the resulting oxygen was spent on the oxidation of iron and sulfur. This continued until all the divalent iron on the surface of the earth was oxidized. From that moment on, oxygen began to accumulate in the atmosphere.
Second half of the Proterozoic – end of the Early Vendian – oxygen concentration in the atmosphere is 0.1% of the current level (Pasteur point).
Late Vendian - Silurian period. Free oxygen stimulated the development of life - the anaerobic fermentation process was replaced by energetically more promising and progressive oxygen metabolism. From this moment on, the accumulation of oxygen in the atmosphere occurred quite quickly. The emergence of plants from the sea onto land (450 million years ago) led to the stabilization of oxygen levels in the atmosphere.
Mid Cretaceous . The final stabilization of oxygen concentration in the atmosphere is associated with the appearance of flowering plants (100 million years ago).
Formation and evolution of nitrogen in the primary and secondary atmosphere of the Earth.
Nitrogen was formed in the early stages of the Earth's development due to the decomposition of ammonia. The fixation of atmospheric nitrogen and its burial in marine sediments began with the appearance of organisms. After living organisms reached land, nitrogen began to be buried in continental sediments. The process of nitrogen fixation especially intensified with the advent of land plants.
Thus, the composition of the Earth’s atmosphere determined the characteristics of the life activity of organisms, contributed to their evolution, development and settlement on the surface of the earth. But in the history of the Earth, there have sometimes been disruptions in the distribution of gas composition. The reason for this was various catastrophes that occurred more than once during the Cryptozoic and Phanerozoic. These failures led to mass extinctions of the organic world.
The composition of the ancient and modern atmosphere of the Earth in percentage terms is given in Table 1.
Table 1. Composition of the primary and modern atmosphere of the Earth.
|
Gases |
Composition of the earth's atmosphere |
|
|
Primary atmosphere, % |
Modern atmosphere, % |
|
| Nitrogen N 2 | ||
| Oxygen O 2 | ||
| Ozone O 3 | ||
| Carbon dioxide CO 2 | ||
| Carbon monoxide CO | ||
| water vapor | ||
| Argon Ar | ||
It was the article “Formation of the Earth's atmosphere. Primary and secondary atmosphere of the Earth." Read further: «
The Earth's primary atmosphere consisted mainly of water vapor, hydrogen and ammonia. Under the influence of ultraviolet radiation from the Sun, water vapor decomposed into hydrogen and oxygen. Hydrogen largely escaped into outer space, oxygen reacted with ammonia and nitrogen and water were formed. At the beginning of geological history, the Earth, thanks to the magnetosphere, which isolated it from the solar wind, created its own secondary carbon dioxide atmosphere. Carbon dioxide came from the depths during intense volcanic eruptions. With the appearance of green plants at the end of the Paleozoic, oxygen began to enter the atmosphere as a result of the decomposition of carbon dioxide during photosynthesis, and the composition of the atmosphere took on its modern form. The modern atmosphere is largely a product of the living matter of the biosphere. Complete renewal of the planet's oxygen by living matter occurs in 5200-5800 years. Its entire mass is absorbed by living organisms in approximately 2 thousand years, all carbon dioxide - in 300-395 years.
Composition of the Earth's primary and modern atmosphere
|
Composition of the earth's atmosphere |
||
|
Upon education* |
Currently |
|
|
Oxygen O 2 |
||
|
Carbon dioxide CO 2 |
||
|
Carbon monoxide CO |
||
|
water vapor |
||
Also present in the primary atmosphere were methane, ammonia, hydrogen, etc. Free oxygen appeared in the atmosphere 1.8-2 billion years ago.
Origin and evolution of the atmosphere (according to V.A. Vronsky and G.V. Voitkovich)
Even during the initial radioactive heating of the young Earth, volatile substances were released to the surface, forming the primary ocean and the primary atmosphere. It can be assumed that the primary atmosphere of our planet was close in composition to the composition of meteorite and volcanic gases. To some extent, the primary atmosphere (CO 2 content was 98%, argon - 0.19%, nitrogen - 1.5%) was similar to the atmosphere of Venus, the planet that is closest in size to our planet.
The Earth's primary atmosphere was of a reducing nature and was practically devoid of free oxygen. Only a small part of it arose in the upper layers of the atmosphere as a result of the dissociation of carbon dioxide and water molecules. Currently, there is a general consensus that at a certain stage in the development of the Earth, its carbon dioxide atmosphere turned into a nitrogen-oxygen atmosphere. However, the question remains unclear regarding the time and nature of this transition - in what era of the history of the biosphere the turning point occurred, whether it was rapid or gradual.
Currently, data have been obtained on the presence of free oxygen in the Precambrian. The presence of highly oxidized iron compounds in the red bands of Precambrian iron ores indicates the presence of free oxygen. The increase in its content throughout the history of the biosphere was determined by constructing appropriate models of varying degrees of reliability (A.P. Vinogradov, G. Holland, J. Walker, M. Shidlovsky, etc.). According to A.P. Vinogradov, the composition of the atmosphere changed continuously and was regulated both by the processes of degassing of the mantle and by physicochemical factors that took place on the Earth’s surface, including cooling and, accordingly, a decrease in ambient temperature. The chemical evolution of the atmosphere and hydrosphere in the past was closely linked in the balance of their substances.
The abundance of buried organic carbon is taken as the basis for calculations of the past composition of the atmosphere, as having passed the photosynthetic stage in the cycle associated with the release of oxygen. With decreasing degassing of the mantle during geological history, the total mass of sedimentary rocks gradually approached modern ones. At the same time, 4/5 of the carbon was buried in carbonate rocks, and 1/5 was accounted for by organic carbon of sedimentary strata. Based on these premises, the German geochemist M. Shidlovsky calculated the increase in the content of free oxygen during the geological history of the Earth. It was found that approximately 39% of all oxygen released during photosynthesis was bound in Fe 2 O 3, 56% was concentrated in SO 4 2 sulfates, and 5% continuously remained in a free state in the Earth’s atmosphere.
In the Early Precambrian, almost all of the released oxygen was quickly absorbed by the earth's crust during oxidation, as well as by volcanic sulfur gases of the primary atmosphere. It is likely that the processes of formation of banded ferruginous quartzites (jaspelites) in the Early and Middle Precambrian led to the absorption of a significant part of the free oxygen from photosynthesis of the ancient biosphere. Ferrous iron in Precambrian seas was the main oxygen absorber when photosynthetic marine organisms supplied free molecular oxygen directly to the aquatic environment. After the Precambrian oceans were cleared of dissolved iron, free oxygen began to accumulate in the hydrosphere and then in the atmosphere.
A new stage in the history of the biosphere was characterized by the fact that in the atmosphere 2000-1800 million years ago there was an increase in the amount of free oxygen. Therefore, the oxidation of iron moved to the surface of ancient continents in the area of the weathering crust, which led to the formation of powerful ancient red-colored strata. The supply of ferrous iron to the ocean has decreased and, accordingly, the absorption of free oxygen by the marine environment has decreased. An increasing amount of free oxygen began to enter the atmosphere, where its constant content was established. In the overall balance of atmospheric oxygen, the role of biochemical processes of living matter in the biosphere has increased. The modern stage in the history of oxygen in the Earth's atmosphere began with the appearance of vegetation on the continents. This led to a significant increase in its content compared to the ancient atmosphere of our planet.
Literature
- Vronsky V.A. Fundamentals of paleogeography / V.A. Vronsky, G.V. Voitkevich. - Rostov n/d: publishing house "Phoenix", 1997. - 576 p.
- Zubaschenko E.M. Regional physical geography. Climates of the Earth: educational and methodological manual. Part 1. / E.M. Zubaschenko, V.I. Shmykov, A.Ya. Nemykin, N.V. Polyakova. – Voronezh: VSPU, 2007. – 183 p.
Formation of the atmosphere. Today, the Earth's atmosphere is a mixture of gases - 78% nitrogen, 21% oxygen and small amounts of other gases, such as carbon dioxide. But when the planet first appeared, there was no oxygen in the atmosphere - it consisted of gases that originally existed in the solar system.
Earth arose when small rocky bodies made of dust and gas from the solar nebula, known as planetoids, collided with each other and gradually took the shape of a planet. As it grew, the gases contained in the planetoids burst out and enveloped the globe. After some time, the first plants began to release oxygen, and the primordial atmosphere developed into the current dense air envelope.
Origin of the atmosphere
- A rain of small planetoids fell on the nascent Earth 4.6 billion years ago. Gases from the solar nebula trapped inside the planet burst out during the collision and formed the Earth's primitive atmosphere, consisting of nitrogen, carbon dioxide and water vapor.
- The heat released during the formation of the planet is retained by a layer of dense clouds in the primordial atmosphere. "Greenhouse gases" such as carbon dioxide and water vapor stop the radiation of heat into space. The surface of the Earth is flooded with a seething sea of molten magma.
- When planetoid collisions became less frequent, the Earth began to cool and oceans appeared. Water vapor condenses from thick clouds, and rain, lasting for several eons, gradually floods the lowlands. Thus the first seas appear.
- The air is purified as water vapor condenses to form oceans. Over time, carbon dioxide dissolves in them, and the atmosphere is now dominated by nitrogen. Due to the lack of oxygen, the protective ozone layer does not form, and ultraviolet rays from the sun reach the earth's surface without hindrance.
- Life appears in ancient oceans within the first billion years. The simplest blue-green algae are protected from ultraviolet radiation by seawater. They use sunlight and carbon dioxide to produce energy, releasing oxygen as a byproduct, which gradually begins to accumulate in the atmosphere.
- Billions of years later, an oxygen-rich atmosphere forms. Photochemical reactions in the upper atmosphere create a thin layer of ozone that scatters harmful ultraviolet light. Life can now emerge from the oceans onto land, where evolution produces many complex organisms.

Billions of years ago, a thick layer of primitive algae began releasing oxygen into the atmosphere. They survive to this day in the form of fossils called stromatolites.

Volcanic origin

1. Ancient, airless Earth. 2. Eruption of gases.
According to this theory, volcanoes were actively erupting on the surface of the young planet Earth. The early atmosphere likely formed when gases trapped in the planet's silicon shell escaped through volcanoes.