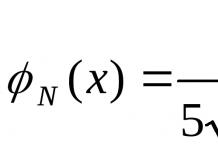Properties of oxides
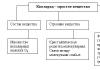
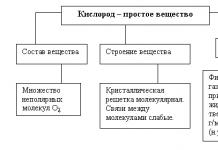
Oxides- these are complex chemical substances, which are chemical compounds of simple elements with oxygen. They are salt-forming And non-salt forming. In this case, there are 3 types of salt-forming agents: main(from the word "foundation"), acidic And amphoteric. An example of oxides that do not form salts are: NO (nitric oxide) - is a colorless, odorless gas. It is formed during a thunderstorm in the atmosphere. CO (carbon monoxide) is an odorless gas produced by the combustion of coal. It is commonly called carbon monoxide. There are other oxides that do not form salts. Now let's take a closer look at each type of salt-forming oxides.
Basic oxides- These are complex chemical substances related to oxides that form salts upon chemical reaction with acids or acidic oxides and do not react with bases or basic oxides. For example, the main ones include the following: K 2 O (potassium oxide), CaO (calcium oxide), FeO (ferrous oxide).
Let's consider chemical properties of oxides with examples
1. Interaction with water: - interaction with water to form a base (or alkali) CaO+H 2 O = Ca(OH) 2 (a known lime slaking reaction, which releases a large amount of heat!)
2. Interaction with acids: - interaction with acid to form salt and water (salt solution in water) CaO + H 2 SO 4 = CaSO 4 + H 2 O (Crystals of this substance CaSO 4 are known to everyone under the name “gypsum”).
3. Interaction with acid oxides: formation of salt CaO + CO 2 = CaCO 3 (Everyone knows this substance - ordinary chalk!)
Acidic oxides- these are complex chemical substances related to oxides that form salts when chemical interaction with bases or basic oxides and do not react with acidic oxides. Examples of acidic oxides can be: CO 2 (well-known carbon dioxide), P 2 O 5 - phosphorus oxide(formed by combustion in air white phosphorus), SO 3 - sulfur trioxide - this substance is used to obtain sulfuric acid.
Chemical reaction with water CO 2 +H 2 O=H 2 CO 3 - this substance is carbonic acid - one of weak acids, it is added to sparkling water to create gas “bubbles”. With increasing temperature, the solubility of gas in water decreases, and its excess comes out in the form of bubbles. - reaction with alkalis (bases): CO 2 +NaOH=Na 2 CO 3 - the resulting substance (salt) is widely used in the household. Its name - soda ash or washing soda - is an excellent detergent for burnt pots, grease, and burnt marks. I do not recommend working with bare hands! - reaction with basic oxides: CO 2 +MgO=MgCO 3 - the resulting salt is magnesium carbonate - also called “bitter salt”.
Amphoteric oxides- these are complex chemical substances, also related to oxides, which form salts during chemical interaction with acids (or acid oxides) and grounds (or basic oxides). The most common use of the word "amphoteric" in our case refers to metal oxides. Example amphoteric oxides can be: ZnO - zinc oxide (white powder, often used in medicine for making masks and creams), Al 2 O 3 - aluminum oxide (also called “alumina”).
The chemical properties of amphoteric oxides are unique in that they can enter into chemical reactions with both bases and acids. For example: - reaction with an acidic oxide: ZnO + H 2 CO 3 = ZnCO 3 + H 2 O - The resulting substance is a solution of “zinc carbonate” salt in water. - reaction with bases: ZnO+2NaOH=Na 2 ZnO 2 +H 2 O - the resulting substance is a double salt of sodium and zinc.
Obtaining oxides produced in various ways. This can happen through physical and chemical means. The simplest way is the chemical interaction of simple elements with oxygen. For example, the result of the combustion process or one of the products of this chemical reaction are oxides. For example, if a hot iron rod, and not only iron (you can take zinc Zn, tin Sn, lead Pb, copper Cu - basically whatever is at hand) is placed in a flask with oxygen, then a chemical reaction of iron oxidation will occur, which accompanied by a bright flash and sparks. The reaction product will be black iron oxide powder FeO: 2Fe+O 2 =2FeO Completely similar chemical reactions with other metals and non-metals, For example: Zinc burns in oxygen to form zinc oxide 2Zn+O 2 =2ZnO The combustion of coal is accompanied by the formation of two oxides at once: carbon monoxide and carbon dioxide 2C+O 2 =2CO - the formation of carbon monoxide. C+O 2 =CO 2 - formation of carbon dioxide. This gas is formed if there is more than enough oxygen, that is, in any case, the reaction first occurs with the formation of carbon monoxide, and then the carbon monoxide is oxidized, turning into carbon dioxide. Obtaining oxides can be done in another way - by chemical decomposition reaction. For example, to obtain iron oxide or aluminum oxide, it is necessary to calcinate the corresponding bases of these metals: Fe(OH) 2 =FeO+H 2 O 2Al(OH) 3 =Al 2 O 3 +3H 2 O, as well as during the decomposition of individual acids: H 2 CO 3 =H 2 O+CO 2 - decomposition of carbonic acid H 2 SO 3 =H 2 O+SO 2 - decomposition of sulfurous acid Obtaining oxides can be carried out from metal salts with strong heating, for example: CaCO 3 = CaO + CO 2 - calcium oxide (or quicklime) and carbon dioxide are obtained by calcining chalk. Cu(NO 3) 2 = 2CuO + 4NO 2 + O 2 - in this decomposition reaction two oxides are obtained at once: copper CuO (black) and nitrogen NO 2 (it is also called brown gas because of its really brown color). Another way in which oxides can be obtained is redox reactions, for example Cu + 4HNO 3 (conc.) = Cu(NO 3) 2 + 2NO 2 + 2H 2 O S + H 2 SO 4 (conc.) = 3SO 2 + 2H 2 O
Chlorine oxides
The following are known chlorine oxides: Cl 2 O, ClO 2, Cl 2 O 6, Cl 2 O 7. All of them, with the exception of Cl 2 O 7, are yellow or orange in color and are not stable, especially ClO 2, Cl 2 O 6. All chlorine oxides are explosive and are very strong oxidizing agents. Reacting with water, they form the corresponding oxygen-containing And chlorine-containing acids: So, Cl 2 O - acid chlorine oxide hypochlorous acid. Cl 2 O + H 2 O = 2HClO - Hypochlorous acid ClO2 - acid chlorine oxide hypochlorous and hypochlorous acid, since during a chemical reaction with water it forms two of these acids at once: ClO 2 + H 2 O = HClO 2 + HClO 3 Cl 2 O 6 - also acid chlorine oxide perchloric and perchloric acids: Cl 2 O 6 + H 2 O = HClO 3 + HClO 4 And finally, Cl 2 O 7 - colorless liquid - acid chlorine oxide perchloric acid: Cl 2 O 7 + H 2 O = HClO 4
Nitrogen oxides
Nitrogen is a gas that forms 5 different compounds with oxygen - 5 nitrogen oxides. Namely: - N 2 O - nitric oxide. Its other name is known in medicine as laughing gas or nitrous oxide- It is colorless, sweetish and pleasant to the taste of gas. - NO - nitrogen monoxide- a colorless, odorless, tasteless gas. - N 2 O 3 - nitrous anhydride- colorless crystalline substance - NO 2 - nitrogen dioxide. Its other name is brown gas- the gas really has a brown-brown color - N 2 O 5 - nitric anhydride- blue liquid, boiling at a temperature of 3.5 0 C
Of all these listed nitrogen compounds, NO - nitrogen monoxide and NO 2 - nitrogen dioxide are of greatest interest in industry. Nitrogen monoxide(NO) and nitrous oxide N 2 O does not react with water or alkalis. Nitrous anhydride(N 2 O 3) when reacting with water forms a weak and unstable nitrous acid HNO 2, which in air gradually turns into a more stable chemical substance nitric acid. Let's look at some chemical properties of nitrogen oxides: Reaction with water: 2NO 2 + H 2 O = HNO 3 + HNO 2 - 2 acids are formed at once: nitric acid HNO 3 and nitrous acid. Reaction with alkali: 2NO 2 + 2NaOH = NaNO 3 + NaNO 2 + H 2 O - two salts are formed: sodium nitrate NaNO 3 (or sodium nitrate) and sodium nitrite (a salt of nitrous acid). Reaction with salts: 2NO 2 + Na 2 CO 3 = NaNO 3 + NaNO 2 + CO 2 - two salts are formed: sodium nitrate and sodium nitrite, and carbon dioxide is released.
Nitrogen dioxide (NO 2) is produced from nitrogen monoxide (NO) using the chemical reaction of compound c oxygen: 2NO + O 2 = 2NO 2
Iron oxides
Iron forms two oxide:FeO- iron oxide(2-valent) - black powder, which is obtained by reduction iron oxide(3-valent) carbon monoxide according to the following chemical reaction: Fe 2 O 3 +CO --> 2FeO+CO 2 This is a basic oxide that easily reacts with acids. It has reducing properties and quickly oxidizes into iron oxide(3-valent). FeO +O 2 --> 2Fe 2 O 3 Iron oxide(3-valent) - red-brown powder (hematite), which has amphoteric properties (can interact with both acids and alkalis). But acid properties of this oxide are so weakly expressed that it is most often used as basic oxide. There are also so-called mixed iron oxide Fe 3 O 4 . It is formed when iron burns and conducts well electricity and has magnetic properties (it is called magnetic iron ore or magnetite). If iron burns, then as a result of the combustion reaction, scale is formed, consisting of two oxides: iron oxide(III) and (II) valence.
August 19, 2012Oxides or oxides are compounds various elements with oxygen. Almost all elements form such compounds. Chlorine, like other halogens, is characterized in such compounds by a positive oxidation state. All chlorine oxides are extremely unstable substances, which is typical for the oxides of all halogens. There are four known substances whose molecules contain chlorine and oxygen.
- A gaseous compound from yellow to reddish color with a characteristic odor (reminiscent of the smell of Cl2 gas) is chlorine oxide (I). Chemical formula Cl2O. Melting point minus 116 °C, boiling point plus 2 °C. At normal conditions its density is 3.22 kg/m³.
- A yellow or yellow-orange gas with a characteristic odor is chlorine oxide (IV). Chemical formula ClO2. Melting point minus 59 °C, boiling point plus 11 °C.
- The red-brown liquid is chlorine oxide (VI). Chemical formula Cl2O6. Melting point plus 3.5 °C, boiling point plus 203 °C.
- Colorless oily liquid - chlorine oxide (VII). Chemical formula Cl2O7. Melting point minus 91.5 °C, boiling point plus 80 °C.
Chlorine oxide with oxidation state +1 is the anhydride of weak monohydric hypochlorous acid (HClO). It is obtained using the Pelouse method by reacting mercury oxide with chlorine gas according to one of the reaction equations: 2Cl2 + 2HgO → Cl2O + Hg2OCl2 or 2Cl2 + HgO → Cl2O + HgCl2. The conditions for these reactions are different. Chlorine oxide (I) condenses at a temperature of minus 60 oC, because at more high temperature it decomposes, exploding, and in concentrated form is explosive. An aqueous solution of Cl2O is obtained by chlorinating alkaline earth or alkali metal carbonates in water. The oxide dissolves well in water, and hypochlorous acid is formed: Cl2O + H2O ↔ 2HClO. In addition, it is also soluble in carbon tetrachloride.
Chlorine oxide with an oxidation state of +4 is otherwise called dioxide. This substance is soluble in water, sulfuric and acetic acids, acetonitrile, carbon tetrachloride, as well as in other organic solvents, with increasing polarity its solubility increases. In laboratory conditions, it is obtained by reacting potassium chlorate with oxalic acid: 2KClO3 + H2C2O4 → K2CO3 + 2ClO2 + CO2 + H2O. Since chlorine oxide (IV) is an explosive substance, it cannot be stored in solution. For these purposes, silica gel is used, on the surface of which ClO2 can be stored in adsorbed form for a long time, while at the same time it is possible to get rid of chlorine contaminants, since it is not absorbed by silica gel. Under industrial conditions, ClO2 is obtained by reduction with sulfur dioxide, in the presence of sulfuric acid, sodium chlorate: 2NaClO3 + SO2 + H2SO4 → 2NaHSO4 + 2ClO2. It is used as a bleaching agent, for example, paper or cellulose, etc., as well as for sterilization and disinfection of various materials.
Chlorine oxide with an oxidation state of +6, upon melting, decomposes according to the reaction equation: Cl2O6 → 2ClO3. Chlorine oxide (VI) is obtained by oxidizing dioxide with ozone: 2O3 + 2ClO2 → 2O2 + Cl2O6. This oxide is capable of interacting with alkali solutions and water. In this case, disproportionation reactions occur. For example, when reacting with potassium hydroxide: 2KOH + Cl2O6 → KClO3 + KClO4 + H2O, the result is potassium chlorate and perchlorate.
Higher chlorine oxide is also called chloric anhydride or dichloroheptaoxide and is a strong oxidizing agent. It can explode on impact or when heated. However, this substance is more stable than oxides with oxidation states +1 and +4. Its decomposition to chlorine and oxygen accelerates due to the presence of lower oxides and with an increase in temperature from 60 to 70 oC. Chlorine oxide (VII) is able to slowly dissolve in cold water; as a result of the reaction, perchloric acid is formed: H2O + Cl2O7 → 2HClO4. Dichloroheptaoxide is obtained by carefully heating perchloric acid with phosphoric anhydride: P4O10 + 2HClO4 → Cl2O7 + H2P4O11. Cl2O7 can also be obtained by using oleum instead of phosphoric anhydride.
Chapter inorganic chemistry, which studies halogen oxides, including chlorine oxides, in last years began to develop actively, since these compounds are energy-intensive. They are capable of delivering energy instantly in the combustion chambers of jet engines, and in chemical sources The current speed of its release can be adjusted. Another reason for interest is the possibility of synthesizing new groups inorganic compounds, for example, chlorine oxide (VII) is the ancestor of perchlorates.
Source: fb.ruCurrent
Author: Chemical Encyclopedia N.S.ZefirovCHLORINE OXIDES. All CHLORINE OXIDES o. have a pungent odor, are thermally and photochemically unstable, prone to explosive decomposition, have a positive Monoxide [Cl(I) oxide, dichloroxide, hemioxide] Cl 2 O is a yellow-orange gas with a faint greenish tint, in liquid state- red-brown; Cl - O bond length 0.1700 nm, angle OClO 111°, 2.60 x 10 -30 Cl x m (table); equation for the temperature dependence of steam pressure logp (mm Hg) = 7.87 - 1373/T (173-288 K); soluble in water to form HNS, solubility (g in 100 g of H 2 O at 0 °C): 33.6 (2.66 kPa), 52.4 (6.65 kPa). At 60-100 °C, the thermodynamic decomposition of Cl 2 O is completed in 12-24 hours; above 110 °C, an explosion occurs after a few minutes; lighting accelerates the decomposition and increases the likelihood of an explosion. With chlorides it forms oxychlorides, for example, with T1Cl 4, TaCl 5 and AsCl 3 it gives T1OCl 2, TaOCl 3 and AsO 2 Cl, respectively. With NO 2 it forms a mixture of NO 2 Cl and NO 3 Cl, with N 2 O 5 - pure NO 3 Cl. Fluorination of Cl 2 O with AgF 2 can produce ClOF 3, and by reaction with AsF 5 or SbF 5 - chloryl salts ClO + 2 MF - 6. ClO 2 and Cl 2 O 6 react similarly with MF 5 (where M is As and Sb). With sat. organic compounds Cl 2 O behaves as a chlorinating agent similar to chlorine. Cl 2 O is prepared by passing Cl 2 diluted with N 2 over HgO or by reacting Cl 2 with wet Na 2 CO 3 .
PROPERTIES OF CHLORINE OXIDES
|
Index |
||||||
|
boiling point, °C |
||||||
|
Density, g/cm 3 |
2.023 (3.5 °C) |
1.805** (25 °C) |
||||
|
J/(mol x K) |
||||||
|
KJ/mol |
||||||
|
KJ/mol |
||||||
|
J/(mol x K) |
*Calculated. **2.38 g/cm 3 at -160 °C.
Dioxide ClO 2 is a yellow gas, in the liquid state it is bright red, in the solid state it is reddish-yellow; C - O bond length 0.1475 nm, OClO angle 117 °C; equation for the temperature dependence of steam pressure logp (mm Hg) = 7.7427 - 1275.1/T (226-312 K); solubility in water 26.1 g/l (25 °C, 20.68 kPa), soluble in CCl 4, HClO 4, CH 3 COOH. In the individual state it is explosive, at 30-50 ° C the decomposition occurs at a measurable rate, above 50 ° C it explodes after an induction period. In an alkaline environment, ClO 2 is disproportionate to and, in the presence. H 2 O 2 is formed and O 2 is released. It is reduced by iodides, arsenides, PbO, H 2 SO 3, amines to chlorite ion. CNO 2 and N 2 O 5 form NO 3 Cl, with NOCl -NO 2 Cl. Fluorinated with AgF 2, BrF 3 or diluted F 2 to ClO 2 F. ClO 2 is obtained by the action of reducing agents (SO 2, NO 2, methanol, organic peroxides) on an acidified solution of alkali metal chlorate, by heating a mixture of chlorate with wet oxalic acid, the action Cl 2 for chlorites. Unlike the rest, CHLORINE OXIDES o. ClO 2 - industrial product. production, it is used instead of Cl 2 as an environmentally safer product for bleaching wood pulp, cellulose, synthetics. fibers, for the preparation of drinking and technol. water, disinfection Wastewater. Irritates mucous membranes, causes coughing, vomiting, etc.; MPC in the air of the working area 0.1 mg/m 3, LD 50 140 mg/kg (rats, intragastric).
Chlorine perchlorate (cichlorotetroxide) Cl 2 O 4, or СlOClО 3 - light yellow liquid, crystalline. state is almost colorless (see Perchlorates).
Trioxide (dichlorohexaoxide) Cl 2 O 6 is a bright red liquid, in the solid state it is orange, the color weakens when cooled. In gases and liquids, molecules have the structure O 2 Cl - O - ClO 3, in crystals they are crystals of the monoclinic system (space group, z = 4); steam pressure 39.9 Pa (0 °C), 133 Pa (19 °C). Slowly decomposes already at 0-10 ° C into ClO 2 and O 2, above 20 ° C Cl 2 appears in the decomposition products; reacts with water with a flash, the products of hydrolysis are HClO 3 and HClO 4. With chlorides, bromides, nitrates it forms perchlorates, for example with NOCl it gives NOClO 4, with N 2 O 5 - NO 2 ClO 4, with AlCl 3 - ClO 2, with FeCl 3 - ClO 2. When heated in a vacuum, such complexes split off Cl 2 O 6 and turn into unsolvated perchlorates Al(ClO 4) 3, Fe(ClO 4) 3. Cl 2 O 6 is obtained by the reaction of ozone with ClO 2 or the action of F 2 on metal chlorates. Used for the synthesis of anhydrous perchlorates in laboratory conditions.
Cl(VII) oxide (chloric anhydride, dichloroheptaoxide) Cl 2 O 7 - colorless. mobile liquid, sensitive to impact and friction. The molecule has the structure O 3 Cl - O - ClO 3, the Cl - O bond length is 0.1709 nm, in ClO 3 groups - 0.1405 nm, ClOCl angle 118.6°, OClO 115.2°, 2.40 x 10 -30 Kl x m; monoclinic crystals (space group C 2/c); equation for the temperature dependence of steam pressure lgp (mm Hg) = 7.796-1770/T. Unlimitedly soluble in CCl 4, highly soluble in HClO 4, POCl 3, etc. It does not mix with water, it reacts at the phase boundary to form HClO 4, the reaction is highly exothermic -211 kJ/mol); heating the Cl 2 O 7 layer can lead to an explosion. The decomposition of Cl 2 O 7 in gas into chlorine and oxygen occurs at a measurable rate at 100-120 ° C, but at a pressure of Cl 2 O 7 above 13.3 kPa it becomes explosive. Liquid Cl 2 O 7 is stable up to 60-70 ° C, an admixture of lower CHLORINE OXIDES o. accelerates its decay. Liquid Cl 2 O 7 is characterized by reactions with the formation of covalent compounds with the group - ClO 3. With NH 3 in CCl 4 it forms NH 4 HNClO 3 and NH 4 ClO 4, with alkylamines - RHNClO 3 and R 2 NClO 3, respectively, with SbF 5 - SbOF 3 and FClO 3, with N 2 O 5 in CCl 4 NO 2 ClO 4 . Using Cl 2 About 7, you can synthesize organic perchlorates from alcohols. Cl 2 O 7 is obtained by the action of P 2 O 5 or oleum on perchloric acid or by electrolysis of a solution of HClO 4 on Pt electrodes below 0 ° C (Cl 2 O 7 accumulates in the anode space). Pure Cl 2 O 7 can also be obtained by heating some perchlorates in a vacuum, for example Nb(ClO 4) 5, MoO 2 (ClO 4) 2.
A number of chlorine-oxygen free radicals are known, obtained in various low-temperature matrices and studied mainly by the EPR method - ClO 3, ClOO, ClClO, as well as the low-stable sesquioxide Cl 2 O 3, which decomposes at -50 - 0 ° C and probably has the structure of chlorine chlorate СlOClO2. Thermally stable radical ClO (Cl - O bond length 0.1569 nm, 4.133 C x m, 101.6 kJ/mol) is an intermediate product of the oxidation of hydrocarbons with perchloric acid and CHLORINE OXIDES o., the decomposition of all CHLORINE OXIDES o. and other chlorine-oxygen compounds, as well as the reaction of ozone with atomic chlorine in the stratosphere.
Literature: Nikitin I.V., Chemistry of oxygen compounds of halogens, M., 1986.
V.Ya.Rosolovsky.
Chemical encyclopedia. Volume 5 >>
Chlorine(I) oxide Cl2O- an endothermic unstable compound can be obtained as follows: 2 Cl 2 + HgO = HgCl 2 + Cl 2 O.
When heated, it decomposes: 2Cl 2 O = 2Cl 2 + O 2, with water it gives hypochlorous acid (has a mild character): Cl 2 O + H 2 O = 2HOCl.
The oxidation state of chlorine is +4. ClO2- chlorine (IV) oxide, endothermic with a pungent odor, has an angular shape, so it is polar.
ClO 2 is characterized by disproportionation reactions: 6ClO 2 + 3H 2 O = 5HClO 3 + HCl,
2ClO 2 + 2KOH = KСlO 2 + KClO 3 + H 2 O. 2KClO 3 + H 2 C 2 O 4 + H 2 SO 4 = K 2 SO 4 + 2CO 2 + 2ClO 2 + 2H 2 O,
Mainly used for bleaching or sterilizing various materials. It has been established that it can be used to dephenolate wastewater from chemical plants.
Cl2O6 gives the disproportionation reaction: 2ClO 2 + 2O 3 = Cl 2 O 6 + 2 O 2,
Cl 2 O 6 + 2 KOH = KClO3 + KClO 4 + H 2 O.
Chlorine(VII) oxide Cl2O7- perchloric acid anhydride HClO 4 (ml polar), relatively stable, when heated (above 120 degrees) it decomposes explosively. 2 HClO 4 + P 2 O 5 = Cl 2 O 7 + 2HPO 3,
Cl 2 O 7 + H 2 O = 2HClO 4, 2Cl 2 O 7 = 2Cl 2 + 7O 2,
Bromine (I) oxide can be obtained as follows: 2 Br 2 + HgO = HgBr 2 + Br2O, at room temperature it
decomposes: 2Br 2 O = 2 Br 2 + O 2.
Bromine (IV) oxide 4O 3 + 3Br 2 = 6BrO 2 is a light yellow solid substance, stable only at -40 degrees. One of the products of its thermal decomposition in a vacuum is brown bromine oxide.
Iodine oxide (V) is obtained by dehydrating iodic acid (with sulfuric acid when heated): 2 HIO 3 = I 2 O 5 + H 2 O, above 3000 C it decomposes: 2 I 2 O 5 = 2 I 2 + 5 O 2.
Question No. 20. Oxygen-containing acids of halogens such as NHO and their salts. Nomenclature. Structure m-l. Sustainability. Oxidative and acidic properties. Bleaching powder. Receipt and application.
Fluorous acid is partially formed by the interaction of a slow flow of fluorine under reduced pressure with cooled water. Isolated only in very small quantities, it is a colorless substance with high pressure vapor, under normal conditions, decomposes quite quickly into HF and O 2. M-la HOF has an angle = 97 degrees. It is apparently strong, but it is quickly hydrolyzed by water, mainly according to the equation: HOF + HOH = HF + H 2 O 2. Its salts have not been obtained, but substances are known, which can be considered as products of the replacement of its hydrogen by radicals of a metalloid character.
Hypochlorous acid very weak, easily decomposes in light with the release of atomic oxygen, which determines its very strong oxidizing properties.
HClO and hypochlorites can be obtained as follows: Cl 2 + H 2 O = HCl + HClO, Cl 2 + 2KOH = KCl + KClO + H 2 O Javel water, Cl 2 + Ca(OH) 2 = CaOCl 2 + H 2 O - chlorine lime Cl 2 O + 2 KOH = 2KClO + H 2 O,
2 HI + HClO = I 2 + HCl + H 2 O. Cl 2 O + H 2 O = 2HOCl.
Hypochlorous acid and hypochlorites are ok. A comparison of standard redox potentials shows that hypochlorous acid is a stronger oxidizing agent than free chlorine and hypochlorites. Large oxidative strength s is explained by the strong polarizable effect of the proton on the chlorine-oxygen bond, in which the bond is deformed and is an unstable formation compared to hypochlorites.
Javel water is used to bleach fabrics, and bleach is used for disinfection.
M-la has an angular structure angle = 103° d(OH) = 0.97, d(ОCl) = 1.69A°.
Hypobromous acid Br 2 + H 2 O = HBr + HBrO, Br 2 + KOH = KBr + KBrO + H 2 O, potassium hypobromite Br 2 + 5 Cl 2 + 6 H 2 O = 2 HBrO + 10 HCl. Potassium hypobromite easily decomposes: 3 KBrO = 2 KBr + KBrO 3 potassium bromate.
Hydrous acid: 2I 2 + HgO + H 2 O = HgI 2 + 2HIO, Salts can be obtained by reacting acids with alkalis or by reactions:
The last 2 compounds are not isolated in the individual state, and the salts - hypobromides and hypoiodides - are quite stable in the absence of oxidation. In this row, the force decreases.
Question No. 21. Oxygen-containing compounds of halogens such as HXO3 and their salts. Nomenclature. The structure of the ml. Sustainability. Oxidizing and acidic properties. Receipt and application. Bertholet's salt. The concept of oscillatory states.
Hypochlorous acid HClO 3 is stable only in aqueous solutions is a strong acid and an energetic oxidizing agent: Ba(ClO 3) 2 + H 2 SO 4 = 2 HClO 3 + BaSO 4, 6P + 5HClO 3 = 3 P 2 O 5 + 5 HCl,
HClO 3 + NaOH = NaClO 3 + H 2 O (sodium chlorate).
As the temperature increases, the reaction occurs: 3 Cl 2 + 6 KOH = 5 KCl + KClO 3 + 3 H 2 O, where KClO 3 is a salt (potassium chlorate), also called Berthollet salt in honor of its discoverer, the French chemist C. Berthollet. It is used as an oxidizing agent in pyrotechnics, in the production of matches, and to produce oxygen in the laboratory. When heated, it decomposes: 4 KClO 3 = KCl + 3 KClO 4, and in the presence of a MnO 2 catalyst, the following occurs: 2 KClO 3 = 2 KCl + 3 O 2.
HBrO 3 - bromic acid (it exists only in solution) can be obtained as follows: Ba(BrO 3) 2 + H 2 SO 4 = 2 HBrO 3 + BaSO 4.
It is interesting to note that iodine can displace bromine from potassium bromate 2 KBrO 3 + I 2 = 2 KIO 3 + Br 2
HIO 3 – iodine (iodates) d(IO) = 1.8 A (two bonds) and 1.9 (one bond) and angle OIO = 98°
I 2 + 5Cl 2 + 6H 2 O = 2HIO 3 + 10HCl, 3I 2 + 10HNO 3 = 6HIO 3 + 10NO + 2H 2 O,
I 2 + 2HClO 3 = 2HIO 3 + Cl 2 (iodine displaces chlorine), IF 5 + 3 H 2 O = 5 HF + HIO 3
Salts can be obtained by reacting acids with alkalis or by reactions:
3 I 2 + 6 NaOH = 5 NaI + NaIO 3 + 3 H 2 O,
The solubility and acid properties of acids decrease, and stability increases