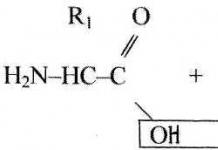
Redox reactions involving organic matter
inclination organic compounds to oxidation is associated with the presence multiple bonds, functional groups, hydrogen atoms at the carbon atom containing the functional group.
Sequential oxidation of organic substances can be represented as the following chain of transformations:
Saturated hydrocarbon → Unsaturated hydrocarbon → Alcohol → Aldehyde (ketone) → Carboxylic acid →CO 2 + H 2 O
The genetic relationship between classes of organic compounds is presented here as a series of oxidative - reducing reactions, providing a transition from one class of organic compounds to another. It is completed by the products of complete oxidation (combustion) of any of the representatives of the classes of organic compounds.
The dependence of the redox ability of organic matter on its structure:
The increased tendency of organic compounds to oxidize is due to the presence of substances in the molecule:
- multiple bonds(that is why alkenes, alkynes, alkadienes are so easily oxidized);
- certain functional groups, capable of being easily oxidized (--SH, -OH (phenolic and alcohol), - NH 2;
- activated alkyl groups located next to multiple bonds. For example, propene can be oxidized to the unsaturated aldehyde acrolein with atmospheric oxygen in the presence of water vapor on bismuth-molybdenum catalysts.
H 2 C═CH−CH 3 → H 2 C═CH−COH
As well as the oxidation of toluene to benzoic acid with potassium permanganate in acidic environment.
5C 6 H 5 CH 3 + 6KMnO 4 + 9H 2 SO 4 → 5C 6 H 5 COOH + 3K 2 SO 4 + 6MnSO 4 + 14H 2 O
- the presence of hydrogen atoms at a carbon atom containing a functional group.
An example is the reactivity in the oxidation reactions of primary, secondary and tertiary alcohols by reactivity to oxidation.
Despite the fact that in the course of any redox reactions, both oxidation and reduction occur, the reactions are classified depending on what happens directly to the organic compound (if it is oxidized, they talk about the oxidation process, if it is reduced, about the reduction process) .
So, in the reaction of ethylene with potassium permanganate, ethylene will be oxidized, and potassium permanganate will be reduced. The reaction is called the oxidation of ethylene.
Application of the concept of "oxidation states" (CO) in organic chemistry is very limited and is realized, first of all, when compiling the equations of redox reactions. However, taking into account that a more or less constant composition of the reaction products is possible only with complete oxidation (combustion) of organic substances, the expediency of arranging the coefficients in the reactions of incomplete oxidation disappears. For this reason, they usually confine themselves to drawing up a scheme for the transformations of organic compounds.
When studying comparative characteristics of inorganic and organic compounds, we got acquainted with the use of the oxidation state (s.o.) (in organic chemistry, primarily carbon) and methods for determining it:
1) calculation of the average s.d. carbon in an organic molecule:
-8/3 +1
This approach is justified if during the reaction in the organic matter all chemical bonds(combustion, complete decomposition).
2) definition of s.o. each carbon atom:
In this case, the oxidation state of any carbon atom in an organic compound is equal to the algebraic sum of the numbers of all bonds with atoms of more electronegative elements, taken into account with the “+” sign at the carbon atom, and the number of bonds with hydrogen atoms (or another more electropositive element), taken into account with the sign "-" at the carbon atom. In this case, bonds with neighboring carbon atoms are not taken into account.
As the simplest example, let's determine the oxidation state of carbon in a methanol molecule.
![]()
The carbon atom is bonded to three hydrogen atoms (these bonds are taken into account with the "-" sign), one bond is with the oxygen atom (it is taken into account with the "+" sign). We get: -3 + 1 = -2. Thus, the oxidation state of carbon in methanol is -2.
The calculated degree of oxidation of carbon, although a conditional value, but it indicates the nature of the shift in the electron density in the molecule, and its change as a result of the reaction indicates an ongoing redox process.
We clarify in which cases it is better to use one or another method.
The processes of oxidation, combustion, halogenation, nitration, dehydrogenation, decomposition are redox processes.
When moving from one class of organic compounds to another andincrease in the degree of branching of the carbon skeleton molecules of compounds within a separate class the oxidation state of the carbon atom responsible for the reducing ability of the compound changes.
Organic substances whose molecules contain carbon atoms with maximum(- and +) CO values(-4, -3, +2, +3), enter into a complete oxidation-combustion reaction, but resistant to mild and medium strength oxidizers.
Substances whose molecules contain carbon atoms in CO -1; 0; +1, oxidize easily, their reduction abilities are close, so their incomplete oxidation can be achieved by one of the known oxidizing agents of low and medium strength. These substances may show dual nature, acting as an oxidizing agent, just as it is inherent in inorganic substances.
When writing the equations for the reactions of combustion and decomposition of organic substances, it is better to use the average value of s.d. carbon.
For example:
![]()
Let's compose complete equation chemical reaction balance method.
The average value of the oxidation state of carbon in n-butane:
The oxidation state of carbon in carbon monoxide (IV) is +4.
Let's make a diagram electronic balance:

Pay attention to the first half of the electronic balance: the carbon atom in the fractional value of s.d. the denominator is 4, so we calculate the transfer of electrons using this coefficient.
Those. going from -2.5 to +4 corresponds to going 2.5 + 4 = 6.5 units. Because 4 carbon atoms are involved, then 6.5 4 \u003d 26 electrons will be given away in total by butane carbon atoms.
Taking into account the found coefficients, the equation for the chemical reaction of n-butane combustion will look like this:
You can use the method for determining the total charge of carbon atoms in a molecule:
(4 C) -10 …… → (1 C) +4 , taking into account that the number of atoms before the = sign and after must be the same, we equalize (4C) -10 …… →[(1 C) +4 ] 4
Therefore, the transition from -10 to +16 is associated with the loss of 26 electrons.
In other cases, we determine the values of s.d. each carbon atom in the compound, while paying attention to the sequence of substitution of hydrogen atoms at primary, secondary, tertiary carbon atoms:


First, the process of substitution occurs at the tertiary, then at the secondary, and, last of all, at the primary carbon atoms.
Alkenes
Oxidation processes depend on the structure of the alkene and the reaction medium.
1. When alkenes are oxidized with a concentrated solution of potassium permanganate KMnO 4 in an acidic environment (hard oxidation) σ- and π-bonds break with the formation of carboxylic acids, ketones, and carbon monoxide (IV). This reaction is used to determine the position of the double bond.
a) If the double bond is at the end of the molecule (for example, in butene-1), then one of the oxidation products is formic acid, which is easily oxidized to carbon dioxide and water:

b) If in the alkene molecule the carbon atom at the double bond contains two carbon substituents (for example, in the molecule of 2-methylbutene-2), then when it is oxidized, a ketone is formed, since the transformation of such an atom into an atom of the carboxyl group is impossible without breaking the C–C bond, which is relatively stable under these conditions:

c) If the alkene molecule is symmetrical and the double bond is contained in the middle of the molecule, then only one acid is formed during oxidation:

A feature of the oxidation of alkenes, in which the carbon atoms in the double bond contain two carbon radicals, is the formation of two ketones:


2. In neutral or slightly alkaline environments, oxidation is accompanied by the formation of diols (dihydric alcohols) , and hydroxyl groups are attached to those carbon atoms between which there was a double bond:

This reaction results in a decolorization of the purple color. aqueous solution KMnO 4 . Therefore, it is used as qualitative reaction into alkenes (Wagner reaction).
3. Oxidation of alkenes in the presence of palladium salts (Wacker process) leads to the formation aldehydes and ketones:
2CH 2 \u003d CH 2 + O 2 PdCl2/H2O→ 2 CH 3 -CO-H
Homologues are oxidized at the less hydrogenated carbon atom:
CH 3 -CH 2 -CH \u003d CH 2 + 1 / 2O 2 PdCl2/H2O→ CH 3 - CH 2 -CO-CH 3
Alkynes
The oxidation of acetylene and its homologues proceeds depending on the medium in which the process takes place.
a) In an acidic environment, the oxidation process is accompanied by the formation of carboxylic acids:

The reaction is used to determine the structure of alkynes by oxidation products:

In neutral and slightly alkaline media, the oxidation of acetylene is accompanied by the formation of the corresponding oxalates (salts of oxalic acid), and the oxidation of homologues is accompanied by the breaking of the triple bond and the formation of salts of carboxylic acids:

For acetylene:
1) In an acidic environment:
H-C≡C-H KMnO 4, H 2 SO 4 → HOOC-COOH (oxalic acid)
3CH≡CH +8KMnO 4 H 2 O→ 3KOOC-COOK potassium oxalate+8MnO 2 ↓+ 2KOH+ 2H 2 O
Arenas
(benzene and its homologues)
When oxidizing arenes in an acidic medium, one should expect the formation of acids, and in an alkaline medium, salts.
Benzene homologues with one side chain (regardless of its length) are oxidized by a strong oxidizing agent to benzoic acid at the α-carbon atom. Benzene homologues, when heated, are oxidized by potassium permanganate in a neutral medium to form potassium salts of aromatic acids.
5C 6 H 5 -CH 3 + 6KMnO 4 + 9H 2 SO 4 \u003d 5C 6 H 5 COOH + 6MnSO 4 + 3K 2 SO 4 + 14H 2 O,
5C 6 H 5 -C 2 H 5 + 12KMnO 4 + 18H 2 SO 4 \u003d 5C 6 H 5 COOH + 5CO 2 + 12MnSO 4 + 6K 2 SO 4 + 28H 2 O,
C 6 H 5 -CH 3 + 2KMnO 4 \u003d C 6 H 5 COOK + 2MnO 2 + KOH + H 2 O.
We emphasize that if there are several side chains in an arene molecule, then in an acidic medium each of them is oxidized at an a-carbon atom to a carboxyl group, resulting in the formation of polybasic aromatic acids:

1) In an acidic environment:
C 6 H 5 -CH 2 -R KMnO 4, H 2 SO 4 → C 6 H 5 -COOH benzoic acid+CO2
2) In a neutral or alkaline environment:
C 6 H 5 -CH 2 -R KMnO4, H2O/(OH)→ C 6 H 5 -COOK + CO 2
3) Oxidation of benzene homologues with potassium permanganate or potassium bichromate when heated:
C 6 H 5 -CH 2 -R KMnO 4, H 2 SO 4, t ˚ C→ C 6 H 5 -COOH benzoic acid+ R-COOH
4) Oxidation of cumene with oxygen in the presence of a catalyst (cumene method for producing phenol):
C 6 H 5 CH(CH 3) 2 O2, H2SO4→ C 6 H 5 -OH phenol + CH 3 -CO-CH 3 acetone
5C 6 H 5 CH(CH 3) 2 + 18KMnO 4 + 27H 2 SO 4 → 5C 6 H 5 COOH + 42H 2 O + 18MnSO 4 + 10CO 2 + K 2 SO 4
C 6 H 5 CH (CH 3) 2 + 6H 2 O - 18'→ C 6 H 5 COOH + 2CO 2 + 18H + | x5
MnO 4 - + 8H + + 5ē→ Mn +2 + 4H 2 O | x18
Attention should be paid to the fact that at mild oxidation of styrene with potassium permanganate KMnO 4 in a neutral or slightly alkaline medium the π-bond breaks, glycol (dihydric alcohol) is formed. As a result of the reaction, the colored solution of potassium permanganate quickly becomes colorless and a brown precipitate of manganese (IV) oxide precipitates.
Oxidation strong oxidizing agent- potassium permanganate in an acidic environment - leads to a complete rupture of the double bond and the formation of carbon dioxide and benzoic acid, the solution becomes colorless.
C 6 H 5 -CH═CH 2 + 2 KMnO 4 + 3 H 2 SO 4 → C 6 H 5 -COOH + CO 2 + K 2 SO 4 + 2 MnSO 4 +4 H 2 O
Alcohols
It should be remembered that:
1) primary alcohols oxidized to aldehydes:
3CH 3 -CH 2 OH + K 2 Cr 2 O 7 + 4H 2 SO 4 \u003d 3CH 3 -CHO + K 2 SO 4 + Cr 2 (SO 4) 3 + 7H 2 O;
2) secondary alcohols are oxidized to ketones:

3) for tertiary alcohols, the oxidation reaction is not typical.
Tertiary alcohols, in the molecules of which there is no hydrogen atom at the carbon atom containing the OH group, do not oxidize under normal conditions. Under severe conditions (under the action of strong oxidizing agents and under high temperatures) they can be oxidized to a mixture of low molecular weight carboxylic acids, i.e. destruction of the carbon skeleton.
When methanol is oxidized with an acidified solution of potassium permanganate or potassium dichromate, CO 2 is formed.
Primary alcohols during oxidation, depending on the reaction conditions, can form not only aldehydes, but also acids.
For example, the oxidation of ethanol with potassium dichromate in the cold ends with the formation of acetic acid, and when heated, acetaldehyde:
3CH 3 -CH 2 OH + 2K 2 Cr 2 O 7 + 8H 2 SO 4 \u003d 3CH 3 -COOH + 2K 2 SO 4 + 2Cr 2 (SO 4) 3 + 11H 2 O,
If three or more OH groups are bonded to adjacent carbon atoms, then the middle or middle atoms are converted to formic acid when oxidized with hydrochloric acid.
The oxidation of glycols with potassium permanganate in an acidic medium proceeds similarly to the oxidative cleavage of alkenes and also leads to the formation of acids or ketones, depending on the structure of the initial glycol.
Aldehydes and ketones
Aldehydes are more easily oxidized than alcohols to the corresponding carboxylic acids not only under the action of strong oxidizing agents (air oxygen, acidified solutions of KMnO 4 and K 2 Cr 2 O 7), but also under the action of weak ones (ammonia solution of silver oxide or copper (II) hydroxide) ):
5CH 3 -CHO + 2KMnO 4 + 3H 2 SO 4 \u003d 5CH 3 -COOH + 2MnSO 4 + K 2 SO 4 + 3H 2 O,
3CH 3 -CHO + K 2 Cr 2 O 7 + 4H 2 SO 4 \u003d 3CH 3 -COOH + Cr 2 (SO 4) 3 + K 2 SO 4 + 4H 2 O,
CH 3 -CHO + 2OH CH 3 -COONH 4 + 2Ag + 3NH 3 + H 2 O
Special attention!!! Oxidation of methanal with an ammonia solution of silver oxide leads to the formation of ammonium carbonate, and not formic acid:
HCHO+ 4OH = (NH 4) 2 CO 3 + 4Ag + 6NH 3 + 2H 2 O.
To compile the equations of redox reactions, both the electron balance method and the half-reaction method (electron-ion method) are used.
For organic chemistry, it is not the oxidation state of the atom that is important, but the shift in electron density, as a result of which partial charges appear on atoms that are in no way consistent with the values of the oxidation states.
Many universities include tickets for entrance exams assignments for the selection of coefficients in the OVR equations by the ion-electron method (half-reaction method). If the school pays at least some attention to this method, it is mainly in the oxidation of inorganic substances.
Let's try to apply the half-reaction method for the oxidation of sucrose with potassium permanganate in an acidic medium.
The advantage of this method is that there is no need to immediately guess and write down the reaction products. They are fairly easy to determine in the course of the equation. An oxidizing agent in an acidic environment most fully manifests its oxidizing properties, for example, the MnO anion - turns into a Mn 2+ cation, easily oxidized organic compounds are oxidized to CO 2.
Let's write in molecular form sucrose conversion:
![]()
On the left side, 13 oxygen atoms are missing; to eliminate this contradiction, let's add 13 H 2 O molecules.
The left side now contains 48 hydrogen atoms, they are released as H + cations:
Now we equalize the total charges on the right and left:
The half-reaction scheme is ready. Drawing up a scheme of the second half-reaction usually does not cause difficulties:
Let's combine both schemes:
![]()
Task for independent work:
Finish UHR and arrange the coefficients using the electronic balance method or the half-reaction method:
CH 3 -CH \u003d CH-CH 3 + KMnO 4 + H 2 SO 4 →
CH 3 -CH \u003d CH-CH 3 + KMnO 4 + H 2O →
(CH 3) 2 C \u003d C-CH 3 + KMnO 4 + H 2 SO 4 →
CH 3 -CH 2 -CH \u003d CH 2 + KMnO 4 + H 2 SO 4 →
WithH 3 -CH 2 -C≡C-CH 3 + KMnO 4 + H 2 SO 4 →
C 6 H 5 -CH 3 + KMnO 4 + H2O →
C 6 H 5 -C 2 H 5 + KMnO 4 + H 2 SO 4 →
C 6 H 5 - CH 3 + KMnO 4 + H 2 SO 4 →
My notes:
Particular attention of students should be paid to the behavior of the oxidizing agent - potassium permanganate KMnO 4 in various environments. This is due to the fact that redox reactions in CMMs occur not only in tasks C1 and C2. In the tasks of SZ, representing a chain of transformations of organic substances, oxidation-reduction equations are not uncommon. At school, the oxidizing agent is often written above the arrow as [O]. The requirement for the performance of such tasks at the USE is the mandatory designation of all starting substances and reaction products with the arrangement of the necessary coefficients.
4.5.b. Oxidative cleavage of alkenes
During the oxidation of alkenes with an alkaline aqueous solution of potassium permanganate when heated or with a solution of KMnO 4 in aqueous sulfuric acid, as well as during the oxidation of alkenes with a solution of chromium (VI) oxide CrO 3 in acetic acid or potassium dichromate and sulfuric acid, the initially formed glycol undergoes oxidative degradation. The end result is the splitting of the carbon skeleton at the site of the double bond and the formation of ketones and/or carboxylic acids as end products, depending on the substituents on the double bond. If both carbon atoms at the double bond contain only one alkyl group, the final product of exhaustive oxidation will be a mixture of carboxylic acids, the tetrasubstituted alkene at the double bond is oxidized to two ketones. Single-substituted alkenes with a terminal double bond are cleaved to carboxylic acid and carbon dioxide.

Due to the low yields of carboxylic acids and ketones, the reactions of exhaustive oxidation of alkenes in the classical version have not found wide application and were previously used mainly to determine the structure of the initial alkene from the products of destructive oxidation. Currently, the oxidation of alkenes (R-CH=CH-R and R-CH=CH 2) to carboxylic acids (RCOOH) with potassium permanganate or dichromate is carried out under conditions of phase transfer catalysis. The yields of carboxylic acids in this case exceed 90%.

4.5.c. Ozonolysis of alkenes
The reaction of alkenes with ozone is the most important method for the oxidative cleavage of alkenes at the double bond. For many decades, this reaction served as the main method for determining the structure of the initial hydrocarbon, and also found application in the synthesis of various carbonyl compounds. The reaction of alkene with ozone is carried out by passing a current of ~5% mixture of ozone and oxygen into a solution of alkene in methylene chloride or ethyl acetate at -80 0 -100 0 C. The end of the reaction is controlled by a test for free ozone with potassium iodide. The mechanism of this peculiar and complex reaction has been established mainly thanks to the work of R. Krige. The first product of the 1,3-dipolar cycloaddition to the double bond is the so-called molozonide (1,2,3-trioxolane). This adduct is unstable and then spontaneously decomposes with ring opening and the formation of normal ozonide (1,2,4-trioxolane) as the final product.

It is now generally accepted that the transformation of molozonide into ordinary ozonide occurs by the splitting-recombination mechanism. Mollozonide undergoes spontaneous opening of the unstable 1,2,3-trioxolane ring with the formation of a carbonyl compound and a bipolar ion, which then react with each other also according to the 1,3-dipolar cycloaddition scheme.

The above scheme of the rearrangement of molozonide into normal ozonide is confirmed by the fact that if before complete education ozonide in the reaction mixture is present as an "interceptor" of the bipolar ion another carbonyl compound, then the so-called "mixed ozonide" is formed. For example, in ozonization cis-stilbene in the presence of benzaldehyde labeled with the 18 O isotope, the label is part of the ether, and not the peroxide bridge of the ozonide:
This result is in good agreement with the formation of a mixed ozonide upon recombination of a bipolar ion with labeled benzaldehyde:

Ozonides are highly unstable compounds that decompose explosively. They are not isolated individually, but split under the action of a wide variety of regents. It is necessary to distinguish between reductive and oxidative cleavage. During hydrolysis, ozonides are slowly split into carbonyl compounds and hydrogen peroxide. Hydrogen peroxide oxidizes aldehydes to carboxylic acids. This is the so-called oxidative decomposition of ozonides:

Thus, during the oxidative decomposition of ozonides, carboxylic acids and (or) ketones are formed, depending on the structure of the initial alkene. Air oxygen, hydrogen peroxide, peracids or silver hydroxide can be used as oxidizing agents. Most often in synthetic practice, hydrogen peroxide in acetic or formic acid, as well as hydrogen peroxide in an alkaline medium, is used for this purpose.


In practice, the method of oxidative decomposition of ozonides is mainly used to obtain carboxylic acids.
More important is the reductive cleavage of ozonides. The most commonly used reducing agents are zinc and acetic acid, triphenylphosphine, or dimethyl sulfide. In this case, the end products of ozonolysis are aldehydes or ketones, depending on the structure of the starting alkene.



From the above examples, it can be seen that a tetra-substituted alkene at a double bond forms two ketones during ozonolysis and subsequent reductive decomposition of the ozonide, while a tri-substituted alkene gives a ketone and an aldehyde. A disubstituted symmetrical alkene forms two aldehydes during ozonolysis, and alkenes with a terminal bond form an aldehyde and formaldehyde.
An interesting modification of ozonolysis is the method where sodium borohydride is used as an ozonide reducing agent. In this case, the final reaction products are primary or secondary alcohols formed during the reduction of aldehydes and xtones, respectively.


Ozonolysis of alkenes is a complex, time-consuming and explosive process that requires the use of special equipment. For this reason, other methods have been developed for the oxidative cleavage of alkenes to carbonyl compounds and carboxylic acids, which successfully replace the ozonolysis reaction in synthetic practice.
One of the modern preparative methods for the oxidative destruction of alkenes was proposed in 1955 by R. Lemieux. This method is based on the hydroxylation of alkenes with potassium permanganate, followed by cleavage of vicinal glycol with sodium periodate NaIO 4 at pH ~ 7 8. Periodate itself does not interact with alkene. The products of this two-step oxidative cleavage are ketones or carboxylic acids, since aldehydes are also oxidized to carboxylic acids under these conditions. In the Lemieux method, the laborious problem of separating one of the reaction products, manganese dioxide, does not arise, since both dioxide and manganate are again oxidized with periodate to a permanganate ion. This allows only catalytic amounts of potassium permanganate to be used. Below are some typical examples oxidative cleavage of alkenes by the Lemieux method.

Citronellol, an alcohol that is part of rose oil, geranium and lemon oils, is oxidized with a mixture of potassium permanganate and sodium periodate in aqueous acetone at 5 10 0 C to 6-hydroxy-4-methylhexanecarboxylic acid with a quantitative yield.

Another variation of this method uses catalytic amounts of osmium tetroxide instead of potassium permanganate (Lemieux & Johnson 1956). A particular advantage of the combination of OsO 4 and NaIO 4 is that it allows the oxidation to be stopped at the aldehyde stage. Osmium tetroxide adds to the double bond of the alkene to form osmate, which is oxidized by sodium periodate to carbonyl compounds with the regeneration of osmium tetroxide.

Instead of osmium tetroxide, ruthenium tetroxide RuO 4 can also be used. Lemieux-Johnson oxidative degradation of alkenes leads to the same products as ozonolysis with reductive cleavage of ozonides.

In terms characteristic of modern organic chemistry, this means that the combination of OsO 4 -NaIO 4 is synthetic equivalent ozonolysis of alkenes followed by reductive cleavage. Similarly, the oxidation of alkenes with a mixture of permanganate and periodate is the synthetic equivalent of ozonolysis with oxidative degradation of ozonides.
Thus, the oxidation of alkenes is not only a set of preparative methods for obtaining alcohols, epoxides, diols, aldehydes, ketones, and carboxylic acids; it is also one of the possible ways to establish the structure of the starting alkene. So, according to the result of the oxidative degradation of the alkene, one can determine the position of the double bond in the molecule, while the stereochemical result syn- or anti- hydroxylation of an alkene makes it possible to draw a conclusion about its geometry.
In redox reactions, organic substances more often exhibit the properties of reducing agents, while they themselves are oxidized. The ease of oxidation of organic compounds depends on the availability of electrons when interacting with an oxidizing agent. All known factors that cause an increase in the electron density in the molecules of organic compounds (for example, positive inductive and mesomeric effects) will increase their ability to oxidize and vice versa.
The tendency of organic compounds to oxidize increases with the growth of their nucleophilicity, which corresponds to the following rows:
The growth of nucleophilicity in the series
Consider redox reactions representatives of the most important classes organic matter with some inorganic oxidizing agents.
Alkene oxidation
With mild oxidation, alkenes are converted to glycols (dihydric alcohols). The reducing atoms in these reactions are carbon atoms linked by a double bond.
The reaction with a solution of potassium permanganate proceeds in a neutral or slightly alkaline medium as follows:
3C 2 H 4 + 2KMnO 4 + 4H 2 O → 3CH 2 OH–CH 2 OH + 2MnO 2 + 2KOH
Under more severe conditions, oxidation leads to the breaking of the carbon chain at the double bond and the formation of two acids (in a strongly alkaline medium, two salts) or an acid and carbon dioxide (in a strongly alkaline medium, a salt and a carbonate):
1) 5CH 3 CH=CHCH 2 CH 3 + 8KMnO 4 + 12H 2 SO 4 → 5CH 3 COOH + 5C 2 H 5 COOH + 8MnSO 4 + 4K 2 SO 4 + 17H 2 O
2) 5CH 3 CH=CH 2 + 10KMnO 4 + 15H 2 SO 4 → 5CH 3 COOH + 5CO 2 + 10MnSO 4 + 5K 2 SO 4 + 20H 2 O
3) CH 3 CH=CHCH 2 CH 3 + 8KMnO 4 + 10KOH → CH 3 COOK + C 2 H 5 COOK + 6H 2 O + 8K 2 MnO 4
4) CH 3 CH \u003d CH 2 + 10KMnO 4 + 13KOH → CH 3 COOK + K 2 CO 3 + 8H 2 O + 10K 2 MnO 4
Potassium dichromate in a sulfuric acid medium oxidizes alkenes similarly to reactions 1 and 2.
During the oxidation of alkenes, in which carbon atoms in the double bond contain two carbon radicals, two ketones are formed:


Alkyne oxidation
Alkynes oxidize under slightly more severe conditions than alkenes, so they usually oxidize with the triple bond breaking the carbon chain. As in the case of alkenes, the reducing atoms here are carbon atoms linked by a multiple bond. As a result of the reactions, acids and carbon dioxide are formed. Oxidation can be carried out with permanganate or potassium dichromate in an acidic environment, for example:
5CH 3 C≡CH + 8KMnO 4 + 12H 2 SO 4 → 5CH 3 COOH + 5CO 2 + 8MnSO 4 + 4K 2 SO 4 + 12H 2 O

Acetylene can be oxidized with potassium permanganate in a neutral medium to potassium oxalate:
3CH≡CH +8KMnO 4 → 3KOOC –COOK +8MnO 2 +2KOH +2H 2 O
In an acidic environment, oxidation goes to oxalic acid or carbon dioxide:
5CH≡CH + 8KMnO 4 + 12H 2 SO 4 → 5HOOC -COOH + 8MnSO 4 + 4K 2 SO 4 + 12H 2 O
CH≡CH + 2KMnO 4 + 3H 2 SO 4 → 2CO 2 + 2MnSO 4 + 4H 2 O + K 2 SO 4
Oxidation of benzene homologues
Benzene does not oxidize even under fairly harsh conditions. Benzene homologues can be oxidized with a solution of potassium permanganate in a neutral medium to potassium benzoate:
C 6 H 5 CH 3 + 2KMnO 4 → C 6 H 5 COOK + 2MnO 2 + KOH + H 2 O
C 6 H 5 CH 2 CH 3 + 4KMnO 4 → C 6 H 5 COOK + K 2 CO 3 + 2H 2 O + 4MnO 2 + KOH
Oxidation of benzene homologues with dichromate or potassium permanganate in an acid medium leads to the formation of benzoic acid.
5C 6 H 5 CH 3 + 6KMnO 4 +9 H 2 SO 4 → 5C 6 H 5 COOH + 6MnSO 4 + 3K 2 SO 4 + 14H 2 O
5C 6 H 5 –C 2 H 5 + 12KMnO 4 + 18H 2 SO 4 → 5C 6 H 5 COOH + 5CO 2 + 12MnSO 4 + 6K 2 SO 4 + 28H 2 O


Alcohol oxidation
The direct products of the oxidation of primary alcohols are aldehydes, while those of secondary alcohols are ketones.
The aldehydes formed during the oxidation of alcohols are easily oxidized to acids; therefore, aldehydes from primary alcohols are obtained by oxidation with potassium dichromate in an acid medium at the boiling point of the aldehyde. Evaporating, aldehydes do not have time to oxidize.
3C 2 H 5 OH + K 2 Cr 2 O 7 + 4H 2 SO 4 → 3CH 3 CHO + K 2 SO 4 + Cr 2 (SO 4) 3 + 7H 2 O
With an excess of an oxidizing agent (KMnO 4, K 2 Cr 2 O 7) in any medium, primary alcohols are oxidized to carboxylic acids or their salts, and secondary alcohols to ketones.
5C 2 H 5 OH + 4KMnO 4 + 6H 2 SO 4 → 5CH 3 COOH + 4MnSO 4 + 2K 2 SO 4 + 11H 2 O
3CH 3 -CH 2 OH + 2K 2 Cr 2 O 7 + 8H 2 SO 4 → 3CH 3 -COOH + 2K 2 SO 4 + 2Cr 2 (SO 4) 3 + 11H 2 O
Tertiary alcohols are not oxidized under these conditions, but methyl alcohol is oxidized to carbon dioxide.

Dihydric alcohol, ethylene glycol HOCH 2 -CH 2 OH, when heated in an acidic medium with a solution of KMnO 4 or K 2 Cr 2 O 7, is easily oxidized to oxalic acid, and in neutral to potassium oxalate.
5CH 2 (OH) - CH 2 (OH) + 8KMnO 4 + 12H 2 SO 4 → 5HOOC -COOH + 8MnSO 4 + 4K 2 SO 4 + 22H 2 O
3CH 2 (OH) - CH 2 (OH) + 8KMnO 4 → 3KOOC -COOK + 8MnO 2 + 2KOH + 8H 2 O
Oxidation of aldehydes and ketones
Aldehydes are rather strong reducing agents, and therefore are easily oxidized by various oxidizing agents, for example: KMnO 4, K 2 Cr 2 O 7, OH, Cu (OH) 2. All reactions take place when heated:
3CH 3 CHO + 2KMnO 4 → CH 3 COOH + 2CH 3 COOK + 2MnO 2 + H 2 O
3CH 3 CHO + K 2 Cr 2 O 7 + 4H 2 SO 4 → 3CH 3 COOH + Cr 2 (SO 4) 3 + 7H 2 O
CH 3 CHO + 2KMnO 4 + 3KOH → CH 3 COOK + 2K 2 MnO 4 + 2H 2 O
5CH 3 CHO + 2KMnO 4 + 3H 2 SO 4 → 5CH 3 COOH + 2MnSO 4 + K 2 SO 4 + 3H 2 O
CH 3 CHO + Br 2 + 3NaOH → CH 3 COONa + 2NaBr + 2H 2 O

silver mirror reaction
With an ammonia solution of silver oxide, aldehydes are oxidized to carboxylic acids, which in ammonia solution give ammonium salts ("silver mirror" reaction):
CH 3 CH \u003d O + 2OH → CH 3 COONH 4 + 2Ag + H 2 O + 3NH 3
CH 3 -CH \u003d O + 2Cu (OH) 2 → CH 3 COOH + Cu 2 O + 2H 2 O
Formic aldehyde (formaldehyde) is oxidized, as a rule, to carbon dioxide:
5HCOH + 4KMnO 4 (hut) + 6H 2 SO 4 → 4MnSO 4 + 2K 2 SO 4 + 5CO 2 + 11H 2 O
3CH 2 O + 2K 2 Cr 2 O 7 + 8H 2 SO 4 → 3CO 2 + 2K 2 SO 4 + 2Cr 2 (SO 4) 3 + 11H 2 O
HCHO + 4OH → (NH 4) 2 CO 3 + 4Ag↓ + 2H 2 O + 6NH 3
HCOH + 4Cu(OH) 2 → CO 2 + 2Cu 2 O↓+ 5H 2 O
Ketones are oxidized under severe conditions by strong oxidizing agents with a gap C-C connections and give mixtures of acids:

carboxylic acids. Among the acids, formic and oxalic acids have strong reducing properties, which are oxidized to carbon dioxide.
HCOOH + HgCl 2 \u003d CO 2 + Hg + 2HCl
HCOOH + Cl 2 \u003d CO 2 + 2HCl
HOOC-COOH + Cl 2 \u003d 2CO 2 + 2HCl
Formic acid, Besides acid properties, also exhibits some properties of aldehydes, in particular, reducing. It is then oxidized to carbon dioxide. For example:
2KMnO4 + 5HCOOH + 3H2SO4 → K2SO4 + 2MnSO4 + 5CO2 + 8H2O
When heated with strong dehydrating agents (H2SO4 (conc.) or P4O10) it decomposes:
HCOOH →(t)CO + H2O
Catalytic oxidation of alkanes:

Catalytic oxidation of alkenes:

Phenol oxidation:


18. Redox reactions (continued 2)
18.9. OVR involving organic substances
In OVR organic substances with inorganic organic substances are most often reducing agents. So, when organic matter burns in excess of oxygen, carbon dioxide and water are always formed. Reactions are more difficult when less active oxidizing agents are used. In this section, only the reactions of representatives of the most important classes of organic substances with some inorganic oxidizing agents are considered.
Alkenes. With mild oxidation, alkenes are converted to glycols (dihydric alcohols). The reducing atoms in these reactions are carbon atoms linked by a double bond.
The reaction with a solution of potassium permanganate proceeds in a neutral or slightly alkaline medium as follows:
C 2 H 4 + 2KMnO 4 + 2H 2 O CH 2 OH–CH 2 OH + 2MnO 2 + 2KOH (cooling)
Under more severe conditions, oxidation leads to the breaking of the carbon chain at the double bond and the formation of two acids (in a strongly alkaline medium, two salts) or an acid and carbon dioxide (in a strongly alkaline medium, a salt and a carbonate):
1) 5CH 3 CH=CHCH 2 CH 3 + 8KMnO 4 + 12H 2 SO 4 5CH 3 COOH + 5C 2 H 5 COOH + 8MnSO 4 + 4K 2 SO 4 + 17H 2 O (heating)
2) 5CH 3 CH=CH 2 + 10KMnO 4 + 15H 2 SO 4 5CH 3 COOH + 5CO 2 + 10MnSO 4 + 5K 2 SO 4 + 20H 2 O (heating)
3) CH 3 CH \u003d CHCH 2 CH 3 + 6KMnO 4 + 10KOH CH 3 COOK + C 2 H 5 COOK + 6H 2 O + 6K 2 MnO 4 (heating)
4) CH 3 CH \u003d CH 2 + 10KMnO 4 + 13KOH CH 3 COOK + K 2 CO 3 + 8H 2 O + 10K 2 MnO 4 (heating)
Potassium dichromate in a sulfuric acid medium oxidizes alkenes similarly to reactions 1 and 2.
Alkynes. Alkynes begin to oxidize under slightly more severe conditions than alkenes, so they usually oxidize with the triple bond breaking the carbon chain. As in the case of alkanes, the reducing atoms here are carbon atoms linked in this case by a triple bond. As a result of the reactions, acids and carbon dioxide are formed. Oxidation can be carried out with permanganate or potassium dichromate in an acidic environment, for example:
5CH 3 C CH + 8KMnO 4 + 12H 2 SO 4 5CH 3 COOH + 5CO 2 + 8MnSO 4 + 4K 2 SO 4 + 12H 2 O (heating)
Sometimes it is possible to isolate intermediate oxidation products. Depending on the position of the triple bond in the molecule, these are either diketones (R 1 –CO–CO–R 2) or aldoketones (R–CO–CHO).
Acetylene can be oxidized with potassium permanganate in a slightly alkaline medium to potassium oxalate:
3C 2 H 2 + 8KMnO 4 \u003d 3K 2 C 2 O 4 + 2H 2 O + 8MnO 2 + 2KOH
In an acidic environment, oxidation goes to carbon dioxide:
C 2 H 2 + 2KMnO 4 + 3H 2 SO 4 \u003d 2CO 2 + 2MnSO 4 + 4H 2 O + K 2 SO 4
Benzene homologues. Benzene homologues can be oxidized with a solution of potassium permanganate in a neutral medium to potassium benzoate:
C 6 H 5 CH 3 + 2KMnO 4 \u003d C 6 H 5 COOK + 2MnO 2 + KOH + H 2 O (at boiling)
C 6 H 5 CH 2 CH 3 + 4KMnO 4 \u003d C 6 H 5 COOK + K 2 CO 3 + 2H 2 O + 4MnO 2 + KOH (when heated)
Oxidation of these substances with dichromate or potassium permanganate in an acidic environment leads to the formation of benzoic acid.
Alcohols. The direct products of the oxidation of primary alcohols are aldehydes, while those of secondary alcohols are ketones.
The aldehydes formed during the oxidation of alcohols are easily oxidized to acids; therefore, aldehydes from primary alcohols are obtained by oxidation with potassium dichromate in an acid medium at the boiling point of the aldehyde. Evaporating, aldehydes do not have time to oxidize.
3C 2 H 5 OH + K 2 Cr 2 O 7 + 4H 2 SO 4 \u003d 3CH 3 CHO + K 2 SO 4 + Cr 2 (SO 4) 3 + 7H 2 O (heating)
With an excess of an oxidizing agent (KMnO 4, K 2 Cr 2 O 7) in any medium, primary alcohols are oxidized to carboxylic acids or their salts, and secondary alcohols to ketones. Tertiary alcohols are not oxidized under these conditions, but methyl alcohol is oxidized to carbon dioxide. All reactions take place when heated.
Dihydric alcohol, ethylene glycol HOCH 2 -CH 2 OH, when heated in an acid medium with a solution of KMnO 4 or K 2 Cr 2 O 7, is easily oxidized to carbon dioxide and water, but sometimes it is possible to isolate intermediate products (HOCH 2 -COOH, HOOC- COOH, etc.).
Aldehydes. Aldehydes are rather strong reducing agents, and therefore are easily oxidized by various oxidizing agents, for example: KMnO 4, K 2 Cr 2 O 7, OH. All reactions take place when heated:
3CH 3 CHO + 2KMnO 4 \u003d CH 3 COOH + 2CH 3 COOK + 2MnO 2 + H 2 O
3CH 3 CHO + K 2 Cr 2 O 7 + 4H 2 SO 4 = 3CH 3 COOH + Cr 2 (SO 4) 3 + 7H 2 O
CH 3 CHO + 2OH \u003d CH 3 COONH 4 + 2Ag + H 2 O + 3NH 3
Formaldehyde with an excess of oxidizing agent is oxidized to carbon dioxide.
18.10. Comparison of the redox activity of various substances
From the definitions of the concepts "oxidizing atom" and "reducing atom" it follows that atoms in the highest oxidation state have only oxidizing properties. On the contrary, only reducing properties are possessed by atoms in the lowest oxidation state. Atoms in intermediate oxidation states can be both oxidizing and reducing agents.
However, based only on the degree of oxidation, it is impossible to unambiguously assess the redox properties of substances. As an example, consider the connections of the elements of the VA group. Nitrogen(V) and antimony(V) compounds are more or less strong oxidizers, bismuth(V) compounds are very strong oxidizers, and phosphorus(V) compounds have practically no oxidizing properties. In this and other similar cases, it matters how much a given oxidation state is characteristic of a given element, that is, how stable compounds containing atoms of a given element in this oxidation state are.
Any OVR proceeds in the direction of the formation of a weaker oxidizing agent and a weaker reducing agent. In the general case, the possibility of any OVR, as well as any other reaction, can be determined by the sign of the change in the Gibbs energy. In addition, to quantify the redox activity of substances, the electrochemical characteristics of oxidizing agents and reducing agents (standard potentials of redox pairs) are used. Based on these quantitative characteristics, it is possible to build series of redox activity of various substances. The series of metal stresses known to you is built in this way. This series makes it possible to compare the reduction properties of metals in aqueous solutions under standard conditions ( with= 1 mol/l, T= 298.15 K), as well as the oxidizing properties of simple aquacations. If ions (oxidizing agents) are placed in the top line of this series, and metal atoms (reducing agents) are placed in the bottom line, then the left side of this series (up to hydrogen) will look like this:
In this series, the oxidizing properties of ions (top line) increase from left to right, while the reducing properties of metals (bottom line), on the contrary, increase from right to left.
Taking into account the differences in redox activity in different media, it is possible to construct similar series for oxidizing agents. So, for reactions in an acidic medium (pH = 0), a "continuation" of a series of metal activity is obtained in the direction of enhancing the oxidizing properties
As in the activity series of metals, in this series the oxidizing properties of oxidizing agents (top row) increase from left to right. But, using this series, it is possible to compare the reducing activity of reducing agents (bottom line) only if their oxidized form coincides with that given in the top line; in this case it amplifies from right to left.
Let's look at a few examples. To find out if this redox is possible, we will use the general rule that determines the direction of the redox reactions (reactions proceed in the direction of the formation of a weaker oxidizing agent and a weaker reducing agent).
1. Can magnesium reduce cobalt from a CoSO 4 solution?
Magnesium is a stronger reducing agent than cobalt, and Co 2 ions are stronger oxidizing agents than Mg 2 ions, therefore, it is possible.
2. Can a solution of FeCl 3 oxidize copper to CuCl 2 in an acidic environment?
Since Fe 3B ions are stronger oxidizing agents than Cu 2 ions, and copper is a stronger reducing agent than Fe 2 ions, it is possible.
3. Is it possible, by blowing oxygen through a solution of FeCl 2 acidified with hydrochloric acid, to obtain a solution of FeCl 3?
It would seem not, since in our series oxygen is to the left of Fe 3 ions and is a weaker oxidizing agent than these ions. But in an aqueous solution, oxygen is almost never reduced to H 2 O 2, in this case it is reduced to H 2 O and occupies a place between Br 2 and MnO 2. Therefore, such a reaction is possible, however, it proceeds rather slowly (why?).
4. Is it possible to oxidize H 2 O 2 in an acidic environment with potassium permanganate?
In this case, H 2 O 2 is a reducing agent and the reducing agent is stronger than Mn 2B ions, and MnO 4 ions are oxidizing agents stronger than oxygen formed from peroxide. Therefore, it is possible.
A similar series constructed for OVR in an alkaline medium looks like this:
Unlike the "acid" series, this series cannot be used in conjunction with the metal activity series.
Electron-ion balance method (half-reaction method), intermolecular OVR, intramolecular OVR, OVR dismutation (disproportionation, self-oxidation-self-healing), OVR switching, passivation.
- Using the method of electron-ion balance, make up the equations of the reactions that occur when a solution of a) H 2 S (S, more precisely, S 8 ) is added to a solution of potassium permanganate acidified with sulfuric acid; b) KHS; c) K 2 S; d) H 2 SO 3; e) KHSO 3 ; e) K 2 SO 3 ; g) HNO 2 ; g) KNO 2 ; i) KI (I 2 ); j) FeSO 4 ; k) C 2 H 5 OH (CH 3 COOH); l) CH 3 CHO; m) (COOH) 2 (CO 2 ); n) K 2 C 2 O 4 . Here and below, where necessary, oxidation products are indicated in curly brackets.
- Make up the equations of the reactions that occur when the following gases are passed through a solution of potassium permanganate acidified with sulfuric acid: a) C 2 H 2 (CO 2 ); b) C 2 H 4 (CO 2 ); c) C 3 H 4 (propyne) (CO 2 and CH 3 COOH); d) C 3 H 6 ; e) CH 4 ; e) HCHO.
- The same, but the reducing agent solution is added to the neutral potassium permanganate solution: a) KHS; b) K 2 S; c) KHSO 3 ; d) K 2 SO 3; e) KNO 2 ; e) KI.
- The same, but potassium hydroxide solution was previously added to the potassium permanganate solution: a) K 2 S (K 2 SO 4 ); b) K 2 SO 3; c) KNO 2 ; d) KI (KIO 3 ).
- Make equations for the following reactions occurring in solution: a) KMnO 4 + H 2 S ...;
b) KMnO 4 + HCl ...;
c) KMnO 4 + HBr ...;
d) KMnO 4 + HI... - Write the following OVR equations for manganese dioxide:
- Solutions of the following substances are added to a solution of potassium dichromate acidified with sulfuric acid: a) KHS; b) K 2 S; c) HNO 2 ; d) KNO 2 ; e) KI; e) FeSO 4 ; g) CH 3 CH 2 CHO; i) H 2 SO 3 ; j) KHSO 3 ; k) K 2 SO 3. Write the equations for the ongoing reactions.
- The same, but the following gases are passed through the solution: a) H 2 S; b) SO2.
- Solutions of a) K 2 S (K 2 SO 4 ) are added to a potassium chromate solution containing potassium hydroxide; b) K 2 SO 3; c) KNO 2 ; d) KI (KIO 3 ). Write the equations for the ongoing reactions.
- A solution of potassium hydroxide was added to a solution of chromium(III) chloride until the initially formed precipitate was dissolved, and then bromine water was added. Write the equations for the ongoing reactions.
- The same, but last step a solution of potassium persulfate K 2 S 2 O 8 was added, recovering during the reaction to sulfate.
- Write the equations for the reactions taking place in the solution:
- Write equations for the reactions that take place between solid chromium trioxide and the following substances: a) C; b) CO; c) S (SO 2 ); d) H 2 S; e) NH 3 ; e) C 2 H 5 OH (CO 2 and H 2 O); g) CH 3 COCH 3 .
- Make up the equations of the reactions that occur when the following substances are added to concentrated nitric acid: a) S (H 2 SO 4 ); b) P 4 ((HPO 3) 4 ); c) graphite; d) Se; e) I 2 (HIO 3 ); e) Ag; g) Cu; i) Pb; j) KF; k) FeO; l) FeS; m) MgO; o) MgS; p) Fe(OH) 2 ; c) P 2 O 3 ; m) As 2 O 3 (H 3 AsO 4 ); y) As 2 S 3; f) Fe(NO 3) 2; x) P 4 O 10 ; c) Cu 2 S.
- The same, but with the passage of the following gases: a) CO; b) H 2 S; c) N 2 O; d) NH3; e) NO; e) H 2 Se; g) HI.
- The reactions will proceed in the same way or differently in the following cases: a) a piece of magnesium was placed in a high test tube two-thirds filled with concentrated nitric acid; b) a drop of concentrated nitric acid was placed on the surface of a magnesium plate? Write reaction equations.
- What is the difference between the reaction of concentrated nitric acid with hydrosulfide acid and with gaseous hydrogen sulfide? Write reaction equations.
- Will OVR proceed in the same way when anhydrous crystalline sodium sulfide and its 0.1 M solution are added to a concentrated solution of nitric acid?
- A mixture of the following substances was treated with concentrated nitric acid: Cu, Fe, Zn, Si and Cr. Write the equations for the ongoing reactions.
- Make up the equations of the reactions that occur when the following substances are added to dilute nitric acid: a) I 2 ; b) Mg; c) Al; d) Fe; e) FeO; f) FeS; g) Fe (OH) 2; i) Fe(OH) 3 ; j) MnS; k) Cu 2 S; l) CuS; m) CuO; n) Na 2 S cr; p) Na 2 S p; c) P 4 O 10 .
- What processes will take place when a) ammonia, b) hydrogen sulfide, c) carbon dioxide is passed through a dilute solution of nitric acid?
- Write the equations for the reactions that occur when added to concentrated sulfuric acid the following substances: a) Ag; b) Cu; c) graphite; d) HCOOH; e) C 6 H 12 O 6; f) NaCl cr; g) C 2 H 5 OH.
- When hydrogen sulfide is passed through cold concentrated sulfuric acid, S and SO 2 are formed, hot concentrated H 2 SO 4 oxidizes sulfur to SO 2. Write reaction equations. How will the reaction proceed between hot concentrated H 2 SO 4 and hydrogen sulfide?
- Why is hydrogen chloride obtained by treating crystalline sodium chloride with concentrated sulfuric acid, while hydrogen bromide and hydrogen iodine are not obtained in this way?
- Make up the equations of the reactions that take place during the interaction of dilute sulfuric acid with a) Zn, b) Al, c) Fe, d) chromium in the absence of oxygen, e) chromium in air.
- Make up the reaction equations that characterize the redox properties of hydrogen peroxide:
- What reactions occur when the following substances are heated: a) (NH 4) 2 CrO 4; b) NaNO 3 ; c) CaCO 3 ; d) Al(NO 3) 3; e) Pb(NO 3) 3 ; f) AgNO 3 ; g) Hg (NO 3) 2; i) Cu(NO 3) 2 ; j) CuO; l) NaClO 4 ; l) Ca(ClO 4) 2; m) Fe(NO 3) 2; n) PCl 5 ; p) MnCl 4 ; c) H 2 C 2 O 4 ; m) LiNO 3 ; s) HgO; f) Ca(NO 3) 2; x) Fe(OH) 3 ; c) CuCl 2 ; h) KClO 3 ; w) KClO 2 ; w) CrO 3 ?
- When hot solutions of ammonium chloride and potassium nitrate are drained, a reaction occurs, accompanied by gas evolution. Write an equation for this reaction.
- Make up the equations of the reactions that occur when a) chlorine is passed through a cold solution of sodium hydroxide, b) bromine vapor. The same, but through a hot solution.
- When interacting with a hot concentrated solution of potassium hydroxide, selenium undergoes dismutation to the nearest stable oxidation states (–II and +IV). Write an equation for this OVR.
- Under the same conditions, sulfur undergoes a similar dismutation, but the excess sulfur reacts with sulfite ions to form thiosulfate ions S 2 O 3 2 . Write the equations for the ongoing reactions. ;
- Make up the equations for the electrolysis reactions of a) a copper nitrate solution with a silver anode, b) a lead nitrate solution with a copper anode.
a) CrCl 2 + FeCl 3; b) CrSO 4 + FeCl 3; c) CrSO 4 + H 2 SO 4 + O 2;
d) CrSO 4 + H 2 SO 4 + MnO 2; e) CrSO 4 + H 2 SO 4 + KMnO 4.
In which of these reactions is hydrogen peroxide an oxidizing agent and in which is it a reducing agent?
| Experience 1. Oxidizing properties of potassium permanganate in an acidic environment. To 3-4 drops of a solution of potassium permanganate add an equal volume of a dilute solution of sulfuric acid, and then a solution of sodium sulfite until discoloration. Write an equation for the reaction. Experience 2.Oxidizing properties potassium permanganate in a neutral medium. Add 5-6 drops of sodium sulfite solution to 3-4 drops of potassium permanganate solution. What substance was isolated in the form of a precipitate? Experience 3. Oxidizing properties of potassium permanganate in an alkaline medium. Add 10 drops of concentrated sodium hydroxide solution and 2 drops of sodium sulfite solution to 3-4 drops of potassium permanganate solution. The solution should turn green. Experience 4. Oxidizing properties of potassium dichromate in an acidic environment. Acidify 6 drops of potassium dichromate solution with 4 drops of dilute sulfuric acid solution and add sodium sulfite solution until the color of the mixture changes. Experience 5. Oxidizing properties of dilute sulfuric acid. Place a zinc granule in one test tube, and a piece of copper tape in the other. Add 8-10 drops of dilute sulfuric acid solution to both tubes. Compare what is happening. EXPERIENCE IN A FAN CABINET! Experience 6. Oxidizing properties of concentrated sulfuric acid. Similar to experiment 5, but add concentrated sulfuric acid solution. A minute after the start of the release of gaseous reaction products, insert strips of filter paper moistened with solutions of potassium permanganate and copper sulfate into the test tubes. Explain what is happening. EXPERIENCE IN A FAN CABINET! Experience 7. Oxidizing properties of dilute nitric acid. Similar to experiment 5, but add a dilute nitric acid solution. Observe the color change of the gaseous reaction products. EXPERIENCE IN A FAN CABINET! Experience 8. Oxidizing properties of concentrated nitric acid. Place a piece of copper tape in a test tube and add 10 drops of concentrated nitric acid solution. Gently heat until the metal is completely dissolved. EXPERIENCE IN A FAN CABINET! Experience 9. Oxidizing properties of potassium nitrite. To 5-6 drops of a solution of potassium nitrite add an equal volume of a dilute solution of sulfuric acid and 5 drops of a solution of potassium iodide. What substances are formed? Experience 10. Reducing properties of potassium nitrite. To 5-6 drops of a solution of potassium permanganate add an equal volume of a dilute solution of sulfuric acid and a solution of potassium nitrite until the mixture is completely discolored. Experience 11.Thermal decomposition of copper nitrate. Place one microspatula of copper nitrate trihydrate in a test tube, fix it in a rack and gently heat it with an open flame. Observe dehydration and subsequent salt decomposition. EXPERIENCE IN A FAN CABINET! Experience 12.Thermal decomposition of lead nitrate. Carry out similarly to experiment 11, placing lead nitrate in a test tube. EXPERIENCE IN A FAN CABINET! What is the difference between the processes occurring during the decomposition of these salts? |
The oxidation of alkenes (acyclic and cyclic) when interacting with peracids (peracids) in a non-polar, indifferent medium is accompanied by the formation of alkene oxides - epoxides, therefore the reaction itself is called the epoxidation reaction.
According to the modern IUPAC nomenclature, the three-membered ring with one oxygen atom is called oxirane.
Epoxidation of alkenes should be considered as a synchronous, coordinated process, which does not involve ionic intermediates such as the OH+ hydroxyl cation. Epoxidation of alkenes is the process of syn-addition of one oxygen atom to a double bond with the complete preservation of the configuration of the substituents at the double bond: 
For epoxidation, a mechanism has been proposed that is characteristic of concerted processes: 
The following peracids are used as epoxidizing agents: perbenzoic, m-chloroperbenzoic, mononaphthalic, peracetic, pertrifluoroacetic, and performic. Aromatic peracids are used as individual reagents, while aliphatic peracids—CH3CO3H, CF3CO3H, and HCO3H—are not isolated individually and are used immediately after their formation by reacting 30% or 90% hydrogen peroxide with the corresponding carboxylic acid. Perbenzoic and meta-chloroperbenzoic acids are currently obtained by oxidation of benzoic and meta-chlorobenzoic acids, respectively, with 70% hydrogen peroxide in a solution of methanesulfonic acid: 
or from acid chlorides and hydrogen peroxide: 
Mononaphthalic acid is obtained by a similar method from phthalic anhydride and 30% hydrogen peroxide in aqueous alkali: 
Initially, perbenzoic or mononaphthalic acids were used to obtain oxiranes (epoxides): 
The method using mononaphthalic acid is especially convenient. Mononaphthalic acid is highly soluble in ether, while one of the reaction products (phthalic acid) is completely insoluble in ether, and it is easy to judge the progress of the reaction by the amount of crystalline phthalic acid released.
Currently, meta-chloroperbenzoic acid is most commonly used for epoxidation. Unlike other peracids, it is stable during storage for a long time (up to 1 year) and absolutely safe to handle. The yields of oxiranes obtained by the oxidation of acyclic and cyclic alkenes with meta-chloroperbenzoic acid in methylene chloride solution are usually very high. 
Peracids are often generated directly in the reaction mixture of 90% hydrogen peroxide and carboxylic acid in methylene chloride: 
Alkenes with a double bond conjugated with a carbonyl and carboxyl group or another acceptor substituent are of low activity, and for their oxidation it is necessary to use stronger oxidizing agents, such as trifluoroperacetic acid obtained from trifluoroacetic acid anhydride and 90% hydrogen peroxide in methylene chloride. An alternative epoxidation method is to react an alkene with a nitrile and 90% hydrogen peroxide: 
The simplest oxirane, ethylene oxide, is produced industrially by the oxidation of ethylene with oxygen in the presence of silver as a catalyst: 
The three-membered ring of oxiranes is easily opened under the action of a wide variety of nucleophilic reagents. These reactions will be discussed in detail in Chapter 11 on acyclic and cyclic reactions. ethers. Here, only the hydrolysis of epoxides will be considered. The hydrolysis of epoxides is catalyzed by both acids and bases. In both cases, vicinal diols are formed, i.e. glycols. During acid catalysis, in the first stage, the protonation of the oxygen atom of the epoxide occurs with the formation of a cyclic oxonium ion, which opens as a result of the nucleophilic attack of the water molecule: 
The key step in ring opening, which determines the rate of the entire process, is the nucleophilic attack of water on the protonated form of the epoxide. From the point of view of the mechanism, this process is similar to the opening of the bromonium ion during the nucleophilic attack of the bromide ion or another nucleophilic agent. From these positions, the stereochemical result should be the formation of trans-glycols during the cleavage of cyclic epoxides. Indeed, in the acid-catalyzed hydrolysis of cyclohexene oxide or cyclopentene oxide, only trans-1,2-diols are formed: 
Thus, the two-stage process of alkene epoxidation followed by acid hydrolysis of the epoxide corresponds in total to the anti-hydroxylation of alkenes.
Both stages of anti-hydroxylation of alkenes can be combined if the alkene is treated with aqueous 30–70% hydrogen peroxide in formic or trifluoroacetic acid. Both of these acids are strong enough to cause epoxy ring opening, so they are commonly used for anti-hydroxylation of alkenes, for example: 
Base-catalyzed epoxy ring opening also leads to the formation of trans-glycols: 
Therefore, the two-stage process of epoxidation of alkenes followed by alkaline hydrolysis of epoxides is also an anti-hydroxylation of alkenes.
Third modern method anti-hydroxylation of alkenes was proposed and developed by C. Prevost (1933). The alkene is heated with iodine and silver benzoate or acetate in anhydrous benzene or CCl4. The trans-addition to the double bond initially leads to the formation of iodoether, in which the iodine is further replaced by the benzoate ion, and glycol dibenzoate is obtained:
The Prevost reaction in an anhydrous medium leads to the formation of the same diol as the epoxidation of alkenes followed by hydrolysis: 
Thus, the Prevost reaction is a more expensive modification of other methods for anti-hydroxylation of alkenes. However, for acid-sensitive compounds, this method has obvious advantages over the method of anti-hydroxylation with peracids and subsequent acid hydrolysis of the epoxide.
Some salts and oxides transition metals higher degrees oxidations are effective reagents for double bond syn-hydroxylation. Oxidation of alkenes with potassium permanganate, one of the oldest methods for double bond syn-hydroxylation, continues to be widely used despite its inherent limitations. cis-1,2-Cyclohexanediol was first obtained by V.V. Markovnikov back in 1878 by hydroxylation of cyclohexene with an aqueous solution of potassium permanganate at 0ºС: 
This method was further developed in the works of the Russian scientist E.E. Wagner, therefore, syn-hydroxylation under the action of an aqueous solution of potassium permanganate is called the Wagner reaction. Potassium permanganate is a strong oxidizing agent capable of not only hydroxylating the double bond, but also cleaving the resulting vicinal diol. In order to avoid further degradation of the glycols whenever possible, the reaction conditions must be carefully controlled. Best Results are achieved by hydroxylation of alkenes in a slightly alkaline medium (pH ~ 8) at 0 - 5ºС with a dilute ~ 1% aqueous solution of KMnO4. However, glycol yields are usually low (30 - 60%): 
Initially, during the oxidation of alkenes with potassium permanganate, a cyclic ester of permanganic acid is formed, which is immediately hydrolyzed to a vicinal diol: 
The cyclic ester of permanganic acid has never been isolated as an intermediate, but its formation follows from experiments with labeled 18O potassium permanganate. K. Weiberg et al. (1957) showed that both oxygen atoms in glycol are labeled during the oxidation of the KMn18O4 alkene. This means that both oxygen atoms are transferred from the oxidizing agent and not from the solvent, water, which is in good agreement with the proposed mechanism.
Another method for the syn-hydroxylation of alkenes under the action of osmium (VIII) oxide OsO4 was proposed by R. Krige in 1936. Osmium tetraoxide is a colorless crystalline substance, readily soluble in ether, dioxane, pyridine, and other organic solvents. When osmium tetroxide reacts with alkenes in ether or dioxane, a black precipitate of the osmic acid cyclic ester is formed - osmate, which can be easily isolated individually. The addition of OsO4 to the double bond is markedly accelerated in a pyridine solution. The decomposition of osmates to vicinal diols is achieved by the action of an aqueous solution of sodium hydrosulfite or hydrogen sulfide: 
The yields of the products of syn-hydroxylation of alkenes in this method are much higher than when using permanganate as an oxidizing agent. An important advantage of the Krige method is the absence of products of oxidative cleavage of alkenes, which is characteristic of permanganate oxidation: 
Osmium tetroxide is an expensive and hard-to-find reagent, besides it is very toxic. Therefore, osmium(VIII) oxide is used for the synthesis of small amounts of hard-to-reach substances in order to obtain the highest diol yield. To simplify the syn-hydroxylation of alkenes under the action of OsO4, a procedure was developed that allows using only catalytic amounts of this reagent. Hydroxylation is carried out with hydrogen peroxide in the presence of OsO4, for example: 
It is interesting to note that higher oxides other transition metals (V2O5, WO3, MoO3, etc.) catalyze the anti-hydroxylation of alkenes.
R. Woodward in 1958 proposed an alternative three-stage method for the syn-hydroxylation of alkenes. Initially, the alkene is converted to trans-iodoacetate by reaction with iodine and silver acetate in acetic acid. Then I replace the halogen with an oxy group when treated with aqueous acetic acid when heated. The last step is the hydrolytic cleavage of the acetate group: 
To conclude this section, we present the stereochemical relationship between the cis- or trans-configuration alkene and the configuration of the resulting vicinal glycol, which can be the cis- or trans-isomer, erythro- or threo-form, meso- or d-,l-form, depending on from substituents in the alkene: 
Similar stereochemical relationships are also observed in other reactions of syn- or anti-addition of hydrogen, hydrogen halides, water, halogens, boron hydrides, and other reagents via a multiple bond.