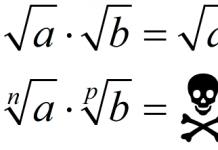Grodno" href="/text/category/grodno/" rel="bookmark">Grodno State Medical University", Candidate of Chemical Sciences, Associate Professor;
Associate Professor of the Department of General and bioorganic chemistry Educational institution "Grodno State Medical University", candidate biological sciences, assistant professor
Reviewers:
Department of General and Bioorganic Chemistry of the Educational Institution “Gomel State Medical University”;
– head Department of Bioorganic Chemistry Educational Institution "Belarusian State Medical University", Candidate of Medical Sciences, Associate Professor.
Department of General and Bioorganic Chemistry of the Educational Institution "Grodno State Medical University"
(minutes dated January 1, 2001)
Central Scientific and Methodological Council of the Educational Institution "Grodno State Medical University"
(minutes dated January 1, 2001)
Section in the specialty 1Medical and psychological affairs of the educational and methodological association of universities of the Republic of Belarus for medical education
(minutes dated January 1, 2001)
Responsible for release:
First Vice-Rector of the Educational Institution "Grodno State Medical University", Professor, Doctor of Medical Sciences
Explanatory note
The relevance of studying the academic discipline
"Bioorganic chemistry"
Bioorganic chemistry is a fundamental natural science discipline. Bioorganic chemistry emerged as an independent science in the 2nd half of the 20th century at the intersection of organic chemistry and biochemistry. The relevance of studying bioorganic chemistry is due to practical tasks, facing medicine and agriculture (obtaining vitamins, hormones, antibiotics, plant growth stimulants, regulators of animal and insect behavior, others medicines), the solution of which is impossible without using the theoretical and practical potential of bioorganic chemistry.
Bioorganic chemistry is constantly being enriched with new methods for the isolation and purification of natural compounds, methods for the synthesis of natural compounds and their analogues, knowledge about the relationship between the structure and biological activity of compounds, etc.
The latest approaches to medical education, related to overcoming the reproductive style in teaching, ensuring cognitive and research activity of students, open up new prospects for realizing the potential of both the individual and the team.
The purpose and objectives of the academic discipline
Target: formation of a level of chemical competence in the medical education system, ensuring subsequent study of biomedical and clinical disciplines.
Tasks:
Students mastering the theoretical foundations of chemical transformations organic molecules in connection with their structure and biological activity;
Formation: knowledge molecular basis vital processes;
Development of skills to navigate classification, structure and properties organic compounds, acting as medicines;
Formation of the logic of chemical thinking;
Development of skills to use qualitative analysis methods
organic compounds;
Chemical knowledge and skills, which form the basis of chemical competence, will contribute to the formation professional competence graduate.
Requirements for mastering an academic discipline
Requirements for the level of mastery of the content of the discipline “Bioorganic Chemistry” are determined educational standard higher education of the first stage in the cycle of general professional and special disciplines, which was developed taking into account the requirements of the competency-based approach, where the minimum content for the discipline is indicated in the form of generalized chemical knowledge and skills that make up the bioorganic competence of a university graduate:
a) generalized knowledge:
- understand the essence of the subject as a science and its connections with other disciplines;
Significance in understanding metabolic processes;
The concept of unity of structure and reactivity organic molecules;
Fundamental laws of chemistry necessary to explain the processes occurring in living organisms;
Chemical properties and the biological significance of the main classes of organic compounds.
b) generalized skills:
Predict the reaction mechanism based on knowledge of the structure of organic molecules and methods of breaking chemical bonds;
Explain the significance of reactions for the functioning of living systems;
Use the acquired knowledge when studying biochemistry, pharmacology and other disciplines.
Structure and content of the academic discipline
In this program, the structure of the content of the discipline “bioorganic chemistry” consists of an introduction to the discipline and two sections that cover general issues of the reactivity of organic molecules, as well as the properties of hetero- and polyfunctional compounds involved in vital processes. Each section is divided into topics arranged in a sequence that ensures optimal learning and assimilation of the program material. For each topic, generalized knowledge and skills are presented that constitute the essence of students’ bioorganic competence. In accordance with the content of each topic, requirements for competencies are determined (in the form of a system of generalized knowledge and skills), for the formation and diagnosis of which tests can be developed.
Teaching methods
The main teaching methods that adequately meet the objectives of studying this discipline are:
Explanation and consultation;
Laboratory lesson;
Elements problem-based learning(educational and research work of students);
Introduction to Bioorganic Chemistry
Bioorganic chemistry is a science that studies the structure of organic substances and their transformations in relation to biological functions. Objects of study of bioorganic chemistry. The role of bioorganic chemistry in the formation of a scientific basis for the perception of biological and medical knowledge at the modern molecular level.
The theory of the structure of organic compounds and its development in modern stage. Isomerism of organic compounds as the basis for the diversity of organic compounds. Types of isomerism of organic compounds.
Physicochemical methods for the isolation and study of organic compounds that are important for biomedical analysis.
Basic Rules systematic nomenclature IUPAC for organic compounds: substitutive and radical-functional nomenclature.
Spatial structure of organic molecules, its connection with the type of hybridization of the carbon atom (sp3-, sp2- and sp-hybridization). Stereochemical formulas. Configuration and conformation. Conformations of open chains (occluded, inhibited, canted). Energy characteristics of conformations. Newman's projection formulas. Spatial proximity of certain sections of the chain as a consequence of conformational equilibrium and as one of the reasons for the predominant formation of five- and six-membered cycles. Conformations of cyclic compounds (cyclohexane, tetrahydropyran). Energy characteristics of chair and bathtub conformations. Axial and equatorial connections. Relationship between spatial structure and biological activity.
Competency requirements:
· Know the objects of study and the main tasks of bioorganic chemistry,
· Be able to classify organic compounds according to the structure of the carbon skeleton and the nature of functional groups, use the rules of systematic chemical nomenclature.
· Know the main types of isomerism of organic compounds, be able to determine possible types of isomers using the structural formula of a compound.
· Know the different types of hybridization of carbon atomic orbitals, the spatial direction of atomic bonds, their type and number depending on the type of hybridization.
· Know the energy characteristics of the conformations of cyclic (chair, bathtub conformations) and acyclic (inhibited, oblique, eclipsed conformations) molecules, be able to depict them using Newman’s projection formulas.
· Know the types of stresses (torsional, angular, van der Waals) that arise in various molecules, their effect on the stability of the conformation and the molecule as a whole.
Section 1. The reactivity of organic molecules as a result of the mutual influence of atoms, mechanisms of organic reactions
Topic 1. Conjugated systems, aromaticity, electronic effects of substituents
Conjugated systems and aromaticity. Conjugation (p, p- and p, p-conjugation). Conjugated open-chain systems: 1,3-dienes (butadiene, isoprene), polyenes (carotenoids, vitamin A). Coupled closed-circuit systems. Aromaticity: criteria for aromaticity, Hückel's rule of aromaticity. Aromaticity of benzenoid (benzene, naphthalene, phenanthrene) compounds. Conjugation energy. Structure and reasons for the thermodynamic stability of carbo- and heterocyclic aromatic compounds. Aromaticity of heterocyclic (pyrrole, imidazole, pyridine, pyrimidine, purine) compounds. Pyrrole and pyridine nitrogen atoms, p-excessive and p-deficient aromatic systems.
Mutual influence of atoms and methods of its transmission in organic molecules. Delocalization of electrons as one of the factors increasing the stability of molecules and ions, its widespread occurrence in biologically important molecules (porphin, heme, hemoglobin, etc.). Polarization of connections. Electronic effects substituents (inductive and mesomeric) as the reason for the uneven distribution of electron density and the emergence of reaction centers in the molecule. Inductive and mesomeric effects (positive and negative), their graphic designation in the structural formulas of organic compounds. Electron-donating and electron-withdrawing substituents.
Competency requirements:
· Know the types of conjugation and be able to determine the type of conjugation based on the structural formula of the compound.
· Know the criteria for aromaticity, be able to determine the aromatic compounds of carbo- and heterocyclic molecules using the structural formula.
· Be able to evaluate the electronic contribution of atoms to the creation of a single conjugated system, know the electronic structure of pyridine and pyrrole nitrogen atoms.
· Know the electronic effects of substituents, the reasons for their occurrence and be able to graphically depict their effect.
· Be able to classify substituents as electron-donating or electron-withdrawing based on the inductive and mesomeric effects they exhibit.
· Be able to predict the effect of substituents on the reactivity of molecules.
Topic 2. Reactivity of hydrocarbons. Radical substitution, electrophilic addition and substitution reactions
General patterns of reactivity of organic compounds as chemical basis their biological functioning. Chemical reaction as a process. Concepts: substrate, reagent, reaction center, transition state, reaction product, activation energy, reaction rate, mechanism.
Classification of organic reactions by result (addition, substitution, elimination, redox) and by mechanism - radical, ionic (electrophilic, nucleophilic), concerted. Types of reagents: radical, acidic, basic, electrophilic, nucleophilic. Homolytic and heterolytic cleavage of covalent bonds in organic compounds and the resulting particles: free radicals, carbocations and carbanions. Electronic and spatial structure of these particles and factors determining their relative stability.
Reactivity of hydrocarbons. Radical substitution reactions: homolytic reactions involving CH bonds of the sp3-hybridized carbon atom. The mechanism of radical substitution using the example of the halogenation reaction of alkanes and cycloalkanes. The concept of chain processes. The concept of regioselectivity.
Pathways for the formation of free radicals: photolysis, thermolysis, redox reactions.
Electrophilic addition reactions ( A.E.) in a row unsaturated hydrocarbons: heterolytic reactions involving p-bonds between sp2-hybridized carbon atoms. Mechanism of hydration and hydrohalogenation reactions. Acid catalysis. Markovnikov's rule. Influence of static and dynamic factors on the regioselectivity of electrophilic addition reactions. Features of electrophilic addition reactions to diene hydrocarbons and small cycles (cyclopropane, cyclobutane).
Electrophilic substitution reactions ( S.E.): heterolytic reactions involving the p-electron cloud of the aromatic system. Mechanism of reactions of halogenation, nitration, alkylation of aromatic compounds: p - and s- complexes. The role of the catalyst (Lewis acid) in the formation of an electrophilic particle.
The influence of substituents in the aromatic ring on the reactivity of compounds in electrophilic substitution reactions. Orienting influence of substituents (orientants of the first and second kind).
Competency requirements:
· Know the concepts of substrate, reagent, reaction center, reaction product, activation energy, reaction rate, reaction mechanism.
· Know the classification of reactions according to various criteria (by the final result, by the method of breaking bonds, by mechanism) and the types of reagents (radical, electrophilic, nucleophilic).
· Know the electronic and spatial structure of reagents and the factors determining their relative stability, be able to compare the relative stability of reagents of the same type.
· Know the methods of formation of free radicals and the mechanism of radical substitution reactions (SR) using examples of halogenation reactions of alkanes and cycloalakane.
· Be able to determine the statistical probability of the formation of possible products in radical substitution reactions and the possibility of regioselective occurrence of the process.
· Know the mechanism of electrophilic addition (AE) reactions in the reactions of halogenation, hydrohalogenation and hydration of alkenes, be able to qualitatively assess the reactivity of substrates based on the electronic effects of substituents.
· Know Markovnikov's rule and be able to determine the regioselectivity of the reactions of hydration and hydrohalogenation based on the influence of static and dynamic factors.
· Know the features of electrophilic addition reactions to conjugated diene hydrocarbons and small cycles (cyclopropane, cyclobutane).
· Know the mechanism of electrophilic substitution reactions (SE) in the reactions of halogenation, nitration, alkylation, acylation of aromatic compounds.
· Be able to determine their influence on reactivity based on the electronic effects of substituents aromatic nucleus and their orienting action.
Topic 3. Acid-base properties of organic compounds
Acidity and basicity of organic compounds: theories of Brønsted and Lewis. The stability of an acid anion is a qualitative indicator of acidic properties. General patterns in changes in acidic or basic properties in connection with the nature of the atoms in the acidic or basic center, the electronic effects of substituents at these centers. Acid properties organic compounds containing hydrogen functional groups(alcohols, phenols, thiols, carboxylic acids, amines, CH-acidity of molecules and cabrications). p-bases and n- grounds. Basic properties of neutral molecules containing heteroatoms with lone pairs of electrons (alcohols, thiols, sulfides, amines) and anions (hydroxide, alkoxide ions, anions of organic acids). Acid-base properties of nitrogen-containing heterocycles (pyrrole, imidazole, pyridine). Hydrogen bonding as a specific manifestation of acid-base properties.
Comparative characteristics of the acidic properties of compounds containing a hydroxyl group (monohydric and polyhydric alcohols, phenols, carboxylic acids). Comparative characteristics of the basic properties of aliphatic and aromatic amines. Influence of the electronic nature of the substituent on the acid-base properties of organic molecules.
Competency requirements:
· Know the definitions of acids and bases according to Bronsted's protolytic theory and Lewis's electron theory.
· Know the Bronsted classification of acids and bases depending on the nature of the atoms of the acidic or basic centers.
· Know the factors influencing the strength of acids and the stability of their conjugate bases, be able to conduct a comparative assessment of the strength of acids based on the stability of their corresponding anions.
· Know the factors influencing the strength of Bronsted bases, be able to conduct a comparative assessment of the strength of the bases taking into account these factors.
· Know the reasons for the occurrence of a hydrogen bond, be able to interpret the formation of a hydrogen bond as a specific manifestation of the acid-base properties of a substance.
· Know the reasons for the occurrence of keto-enol tautomerism in organic molecules, be able to explain them from the perspective of the acid-base properties of compounds in connection with their biological activity.
· Know and be able to carry out qualitative reactions, allowing to distinguish polyhydric alcohols, phenols, thiols.
Topic 4. Nucleophilic substitution reactions at the tetragonal carbon atom and competitive elimination reactions
Nucleophilic substitution reactions at the sp3-hybridized carbon atom: heterolytic reactions caused by polarization of the carbon-heteroatom bond (halogen derivatives, alcohols). Groups that leave easily and difficultly: the connection between the ease of leaving a group and its structure. The influence of solvent, electronic and spatial factors on the reactivity of compounds in reactions of mono- and bimolecular nucleophilic substitution (SN1 and SN2). Stereochemistry of nucleophilic substitution reactions.
Hydrolysis reactions of halogen derivatives. Alkylation reactions of alcohols, phenols, thiols, sulfides, ammonia, amines. The role of acid catalysis in nucleophilic substitution of the hydroxyl group. Halogen derivatives, alcohols, esters of sulfuric and phosphoric acids as alkylating reagents. Biological role of alkylation reactions.
Mono- and bimolecular elimination reactions (E1 and E2): (dehydration, dehydrohalogenation). Increased CH acidity as a cause of elimination reactions accompanying nucleophilic substitution at the sp3-hybridized carbon atom.
Competency requirements:
· Know the factors that determine the nucleophilicity of reagents and the structure of the most important nucleophilic particles.
· Know the general laws of nucleophilic substitution reactions at a saturated carbon atom, the influence of static and dynamic factors on the reactivity of a substance in a nucleophilic substitution reaction.
· Know the mechanisms of mono- and bimolecular nucleophilic substitution, be able to evaluate the influence of steric factors, the influence of solvents, the influence of static and dynamic factors on the course of a reaction according to one of the mechanisms.
· Know the mechanisms of mono- and bimolecular elimination, the reasons for competition between nucleophilic substitution and elimination reactions.
· Know Zaitsev's rule and be able to determine the main product in the reactions of dehydration and dehydrohalogenation of unsymmetrical alcohols and haloalkanes.
Topic 5. Reactions of nucleophilic addition and substitution at the trigonal carbon atom
Nucleophilic addition reactions: heterolytic reactions involving the carbon-oxygen p-bond (aldehydes, ketones). The mechanism of reactions of interaction of carbonyl compounds with nucleophilic reagents (water, alcohols, thiols, amines). Influence of electronic and spatial factors, the role of acid catalysis, reversibility of nucleophilic addition reactions. Hemiacetals and acetals, their preparation and hydrolysis. Biological role of acetalization reactions. Aldol addition reactions. Basic catalysis. Structure of the enolate ion.
Nucleophilic substitution reactions in the series of carboxylic acids. Electronic and spatial structure of the carboxyl group. Nucleophilic substitution reactions at the sp2-hybridized carbon atom (carboxylic acids and their functional derivatives). Acylating agents (acid halides, anhydrides, carboxylic acids, esters, amides), Comparative characteristics their reactivity. Acylation reactions - the formation of anhydrides, esters, thioesters, amides - and their reverse hydrolysis reactions. Acetyl coenzyme A is a natural high-energy acylating agent. Biological role of acylation reactions. The concept of nucleophilic substitution at phosphorus atoms, phosphorylation reactions.
Oxidation and reduction reactions of organic compounds. Specificity of redox reactions of organic compounds. The concept of one-electron transfer, hydride ion transfer and the action of the NAD+ ↔ NADH system. Oxidation reactions of alcohols, phenols, sulfides, carbonyl compounds, amines, thiols. Reduction reactions of carbonyl compounds and disulfides. The role of redox reactions in life processes.
Competency requirements:
· Know the electronic and spatial structure of the carbonyl group, the influence of electronic and steric factors on the reactivity of the oxo group in aldehydes and ketones.
· Know the mechanism of reactions of nucleophilic addition of water, alcohols, amines, thiols to aldehydes and ketones, the role of a catalyst.
· Know the mechanism of aldol condensation reactions, the factors determining the participation of a compound in this reaction.
· Know the mechanism of reduction reactions of oxo compounds with metal hydrides.
· Know reaction centers, present in carboxylic acid molecules. Be able to conduct a comparative assessment of the strength of carboxylic acids depending on the structure of the radical.
· Know the electronic and spatial structure of the carboxyl group, be able to conduct a comparative assessment of the ability of the carbon atom of the oxo group in carboxylic acids and their functional derivatives (acid halides, anhydrides, esters, amides, salts) to undergo nucleophilic attack.
· Know the mechanism of nucleophilic substitution reactions using examples of acylation, esterification, hydrolysis of esters, anhydrides, acid halides, amides.
Topic 6. Lipids, classification, structure, properties
Lipids, saponifiable and unsaponifiable. Neutral lipids. Natural fats as a mixture of triacylglycerols. The main natural higher fatty acids that make up lipids: palmitic, stearic, oleic, linoleic, linolenic. Arachidonic acid. Features of unsaturated fatty acids, w-nomenclature.
Peroxide oxidation of unsaturated fatty acid fragments in cell membranes. The role of membrane lipid peroxidation in the effect of low doses of radiation on the body. Antioxidant protection systems.
Phospholipids. Phosphatidic acids. Phosphatidylcolamines and phosphatidylserines (cephalins), phosphatidylcholines (lecithins) are structural components of cell membranes. Lipid bilayer. Sphingolipids, ceramides, sphingomyelins. Brain glycolipids (cerebrosides, gangliosides).
Competency requirements:
· Know the classification of lipids and their structure.
· Know the structure structural components saponified lipids - alcohols and higher fatty acids.
· Know the mechanism of reactions of formation and hydrolysis of simple and complex lipids.
· Know and be able to carry out qualitative reactions to unsaturated fatty acids and oils.
· Know the classification of unsaponifiable lipids, have an idea of the principles of classification of terpenes and steroids, their biological role.
· Know the biological role of lipids, their main functions, have an idea of the main stages peroxidation lipids and the consequences of this process for the cell.
Section 2. Stereoisomerism of organic molecules. Poly- and heterofunctional compounds involved in vital processes
Topic 7. Stereoisomerism of organic molecules
Stereoisomerism in a series of compounds with a double bond (p-diastereomerism). Cis and trans isomerism of unsaturated compounds. E, Z – notation system for p-diastereomers. Comparative stability of p-diastereomers.
Chiral molecules. Asymmetric carbon atom as a center of chirality. Stereoisomerism of molecules with one center of chirality (enantiomerism). Optical activity. Fischer projection formulas. Glyceraldehyde as a configuration standard, absolute and relative configuration. D, L-system of stereochemical nomenclature. R, S-system of stereochemical nomenclature. Racemic mixtures and methods for their separation.
Stereoisomerism of molecules with two or more chiral centers. Enantiomers, diastereomers, mesoforms.
Competency requirements:
· Know the reasons for the occurrence of stereoisomerism in the series of alkenes and diene hydrocarbons.
· Be able to use the abbreviated structural formula of an unsaturated compound to determine the possibility of the existence of p-diastereomers, distinguish between cis - trans isomers, and evaluate their comparative stability.
· Know the symmetry elements of molecules, the necessary conditions for the appearance of chirality in an organic molecule.
· Know and be able to represent enantiomers using projection formulas Fischer, calculate the number of expected stereoisomers based on the number of chiral centers in the molecule, principles for determining the absolute and relative configuration, D-, L-system of stereochemical nomenclature.
· Know the methods for separating racemates, the basic principles of the R, S-system of stereochemical nomenclature.
Topic 8. Physiologically active poly- and heterofunctional compounds of the aliphatic, aromatic and heterocyclic series
Poly- and heterofunctionality as one of the characteristic features of organic compounds participating in vital processes and being the ancestors of the most important groups of medicines. Peculiarities in the mutual influence of functional groups depending on their relative location.
Polyhydric alcohols: ethylene glycol, glycerin. Esters polyhydric alcohols with inorganic acids (nitroglycerin, glycerol phosphates). Diatomic phenols: hydroquinone. Oxidation of diatomic phenols. Hydroquinone-quinone system. Phenols as antioxidants (free radical scavengers). Tocopherols.
Dibasic carboxylic acids: oxalic, malonic, succinic, glutaric, fumaric. The conversion of succinic acid to fumaric acid is an example of a biologically important dehydrogenation reaction. Decarboxylation reactions, their biological role.
Amino alcohols: aminoethanol (colamine), choline, acetylcholine. The role of acetylcholine in the chemical transmission of nerve impulses at synapses. Aminophenols: dopamine, norepinephrine, adrenaline. The concept of the biological role of these compounds and their derivatives. Neurotoxic effects of 6-hydroxydopamine and amphetamines.
Hydroxy and amino acids. Cyclization reactions: the influence of various factors on the process of cycle formation (implementation of the corresponding conformations, size of the resulting cycle, entropy factor). Lactones. Lactams. Hydrolysis of lactones and lactams. Elimination reaction of b-hydroxy and amino acids.
Aldehyde and keto acids: pyruvic, acetoacetic, oxaloacetic, a-ketoglutaric. Acid properties and reactivity. Reactions of decarboxylation of b-keto acids and oxidative decarboxylation of a-keto acids. Acetoacetic ester, keto-enol tautomerism. Representatives of “ketone bodies” are b-hydroxybutyric acid, b-ketobutyric acid, acetone, their biological and diagnostic significance.
Heterofunctional benzene derivatives as medicines. Salicylic acid and its derivatives (acetylsalicylic acid).
Para-aminobenzoic acid and its derivatives (anesthesin, novocaine). Biological role of p-aminobenzoic acid. Sulfanilic acid and its amide (streptocide).
Heterocycles with several heteroatoms. Pyrazole, imidazole, pyrimidine, purine. Pyrazolone-5 is the basis of non-narcotic analgesics. Barbituric acid and its derivatives. Hydroxypurines (hypoxanthine, xanthine, uric acid), their biological role. Heterocycles with one heteroatom. Pyrrole, indole, pyridine. Biologically important pyridine derivatives are nicotinamide, pyridoxal, and isonicotinic acid derivatives. Nicotinamide is a structural component of the coenzyme NAD+, which determines its participation in OVR.
Competency requirements:
· Be able to classify heterofunctional compounds by composition and by their relative arrangement.
· Know specific reactions amino and hydroxy acids with a, b, g - arrangement of functional groups.
· Know the reactions leading to the formation of biologically active compounds: choline, acetylcholine, adrenaline.
· Know the role of keto-enol tautomerism in the manifestation of the biological activity of keto acids (pyruvic acid, oxaloacetic acid, acetoacetic acid) and heterocyclic compounds (pyrazole, barbituric acid, purine).
· Know the methods of redox transformations of organic compounds, the biological role of redox reactions in the manifestation of the biological activity of diatomic phenols, nicotinamide, and the formation of ketone bodies.
Subject9 . Carbohydrates, classification, structure, properties, biological role
Carbohydrates, their classification in relation to hydrolysis. Classification of monosaccharides. Aldoses, ketoses: trioses, tetroses, pentoses, hexoses. Stereoisomerism of monosaccharides. D- and L-series of stereochemical nomenclature. Open and cyclic forms. Fisher's formulas and Haworth's formulas. Furanoses and pyranoses, a- and b-anomers. Cyclo-oxo-tautomerism. Conformations of pyranose forms of monosaccharides. The structure of the most important representatives of pentoses (ribose, xylose); hexoses (glucose, mannose, galactose, fructose); deoxysugars (2-deoxyribose); amino sugars (glucosamine, mannosamine, galactosamine).
Chemical properties of monosaccharides. Nucleophilic substitution reactions involving an anomeric center. O - and N-glycosides. Hydrolysis of glycosides. Phosphates of monosaccharides. Oxidation and reduction of monosaccharides. Reducing properties of aldoses. Glyconic, glycaric, glycuronic acids.
Oligosaccharides. Disaccharides: maltose, cellobiose, lactose, sucrose. Structure, cyclo-oxo-tautomerism. Hydrolysis.
Polysaccharides. General characteristics and classification of polysaccharides. Homo- and heteropolysaccharides. Homopolysaccharides: starch, glycogen, dextrans, cellulose. Primary structure, hydrolysis. The concept of secondary structure (starch, cellulose).
Competency requirements:
· Know the classification of monosaccharides (according to the number of carbon atoms, the composition of functional groups), the structure of open and cyclic forms (furanose, pyranose) of the most important monosaccharides, their ratio of D - and L - series of stereochemical nomenclature, be able to determine the number of possible diastereomers, classify stereoisomers as diastereomers , epimers, anomers.
· Know the mechanism of cyclization reactions of monosaccharides, the reasons for the mutarotation of monosaccharide solutions.
· Know the chemical properties of monosaccharides: redox reactions, reactions of formation and hydrolysis of O - and N-glycosides, esterification reactions, phosphorylation.
· Be able to carry out high-quality reactions on the diol fragment and the presence of reducing properties of monosaccharides.
· Know the classification of disaccharides and their structure, the configuration of the anomeric carbon atom forming a glycosidic bond, tautomeric transformations of disaccharides, their chemical properties, biological role.
· Know the classification of polysaccharides (in relation to hydrolysis, according to monosaccharide composition), the structure of the most important representatives of homopolysaccharides, the configuration of the anomeric carbon atom forming a glycosidic bond, their physical and chemical properties, and biological role. Have an idea of the biological role of heteropolysaccharides.
Topic 10.a-Amino acids, peptides, proteins. Structure, properties, biological role
Structure, nomenclature, classification of a-amino acids that make up proteins and peptides. Stereoisomerism of a-amino acids.
Biosynthetic pathways for the formation of a-amino acids from oxoacids: reductive amination reactions and transamination reactions. Essential amino acids.
Chemical properties of a-amino acids as heterofunctional compounds. Acid-base properties of a-amino acids. Isoelectric point, methods for separating a-amino acids. Formation of intracomplex salts. Reactions of esterification, acylation, alkylation. Interaction nitrous acid and formaldehyde, the significance of these reactions for the analysis of amino acids.
g-Aminobutyric acid is an inhibitory neurotransmitter of the central nervous system. Antidepressant effect of L-tryptophan, serotonin - as a sleep neurotransmitter. Mediator properties of glycine, histamine, aspartic and glutamic acids.
Biologically important reactions a-amino acids. Deamination and hydroxylation reactions. Decarboxylation of a-amino acids is the path to the formation of biogenic amines and bioregulators (colamine, histamine, tryptamine, serotonin.) Peptides. Electronic structure peptide bond. Acid and alkaline hydrolysis of peptides. Establishment of amino acid composition using modern physical and chemical methods(Sanger and Edman methods). Concept of neuropeptides.
Primary structure of proteins. Partial and complete hydrolysis. The concept of secondary, tertiary and quaternary structures.
Competency requirements:
· Know the structure, stereochemical classification of a-amino acids, belonging to the D- and L-stereochemical series of natural amino acids, essential amino acids.
· Know the ways of synthesis of a-amino acids in vivo and in vitro, know the acid-base properties and methods of converting a-amino acids into an isoelectric state.
· Know the chemical properties of a-amino acids (reactions on amino and carboxyl groups), be able to carry out qualitative reactions (xantoprotein, with Cu(OH)2, ninhydrin).
· Know the electronic structure of the peptide bond, the primary, secondary, tertiary and quaternary structure of proteins and peptides, know how to determine the amino acid composition and amino acid sequence (Sanger method, Edman method), be able to carry out the biuret reaction for peptides and proteins.
· Know the principle of the method of peptide synthesis using protection and activation of functional groups.
Topic 11. Nucleotides and nucleic acids
Nucleic bases included in the composition nucleic acids. Pyrimidine (uracil, thymine, cytosine) and purine (adenine, guanine) bases, their aromaticity, tautomeric transformations.
Nucleosides, reactions of their formation. The nature of the connection between the nucleic base and the carbohydrate residue; configuration of the glycosidic center. Hydrolysis of nucleosides.
Nucleotides. The structure of mononucleotides that form nucleic acids. Nomenclature. Hydrolysis of nucleotides.
Primary structure of nucleic acids. Phosphodiester bond. Ribonucleic and deoxyribonucleic acids. Nucleotide composition of RNA and DNA. Hydrolysis of nucleic acids.
The concept of the secondary structure of DNA. The role of hydrogen bonds in the formation of secondary structure. Complementarity of nucleic bases.
Medicines based on modified nucleic bases (5-fluorouracil, 6-mercaptopurine). The principle of chemical similarity. Changes in the structure of nucleic acids under the influence of chemicals and radiation. Mutagenic effect of nitrous acid.
Nucleoside polyphosphates (ADP, ATP), features of their structure that allow them to perform the functions of high-energy compounds and intracellular bioregulators. The structure of cAMP, the intracellular “messenger” of hormones.
Competency requirements:
· Know the structure of pyrimidine and purine nitrogenous bases, their tautomeric transformations.
· Know the mechanism of reactions for the formation of N-glycosides (nucleosides) and their hydrolysis, the nomenclature of nucleosides.
· Know the fundamental similarities and differences between natural and synthetic antibiotic nucleosides in comparison with the nucleosides that make up DNA and RNA.
· Know the reactions of nucleotide formation, the structure of mononucleotides that make up nucleic acids, their nomenclature.
· Know the structure of cyclo- and polyphosphates of nucleosides, their biological role.
· Know the nucleotide composition of DNA and RNA, the role of the phosphodiester bond in creating the primary structure of nucleic acids.
· Know the role of hydrogen bonds in the formation of the secondary structure of DNA, the complementarity of nitrogenous bases, the role of complementary interactions in the implementation of the biological function of DNA.
· Know the factors that cause mutations and the principle of their action.
Information part
Bibliography
Main:
1. Romanovsky, bioorganic chemistry: a textbook in 2 parts /. - Minsk: BSMU, 20с.
2. Romanovsky, to the workshop on bioorganic chemistry: tutorial/ edited by. – Minsk: BSMU, 1999. – 132 p.
3. Tyukavkina, N. A., Bioorganic chemistry: textbook / , . – Moscow: Medicine, 1991. – 528 p.
Additional:
4. Ovchinnikov, chemistry: monograph /.
– Moscow: Education, 1987. – 815 p.
5. Potapov: textbook /. - Moscow:
Chemistry, 1988. – 464 p.
6. Riles, A. Fundamentals of organic chemistry: a textbook / A. Rice, K. Smith,
R. Ward. – Moscow: Mir, 1989. – 352 p.
7. Taylor, G. Fundamentals of organic chemistry: textbook / G. Taylor. -
Moscow: Mirs.
8. Terney, A. Modern organic chemistry: a textbook in 2 volumes /
A. Terney. – Moscow: Mir, 1981. – 1310 p.
9. Tyukavkina, for laboratory classes on bioorganic
chemistry: textbook / [etc.]; edited by N.A.
Tyukavkina. – Moscow: Medicine, 1985. – 256 p.
10. Tyukavkina, N. A., Bioorganic chemistry: A textbook for students
medical institutes / , . - Moscow.
Plan 1. Subject and significance of bioorganic chemistry 2. Classification and nomenclature of organic compounds 3. Methods of depicting organic molecules 4. Chemical bond in bioorganic molecules 5. Electronic effects. Mutual influence of atoms in a molecule 6. Classification chemical reactions and reagents 7. Concept of the mechanisms of chemical reactions 2

Subject of bioorganic chemistry 3 Bioorganic chemistry independent section chemical science, which studies the structure, properties and biological functions of chemical compounds of organic origin that take part in the metabolism of living organisms.

The objects of study of bioorganic chemistry are low-molecular biomolecules and biopolymers (proteins, nucleic acids and polysaccharides), bioregulators (enzymes, hormones, vitamins and others), natural and synthetic physiologically active compounds, including drugs and substances with toxic effects. Biomolecules are bioorganic compounds that are part of living organisms and specialized for the formation cellular structures and participation in biochemical reactions, form the basis of metabolism (metabolism) and physiological functions of living cells and multicellular organisms in general. 4 Classification of bioorganic compounds

Metabolism is a set of chemical reactions that occur in the body (in vivo). Metabolism is also called metabolism. Metabolism can occur in two directions - anabolism and catabolism. Anabolism is the synthesis in the body of complex substances from relatively simple ones. It occurs with the expenditure of energy (endothermic process). Catabolism, on the contrary, is the breakdown of complex organic compounds into simpler ones. It occurs with the release of energy (exothermic process). Metabolic processes take place with the participation of enzymes. Enzymes play the role of biocatalysts in the body. Without enzymes, biochemical processes would either not occur at all, or would proceed very slowly, and the body would not be able to maintain life. 5

Bioelements. The composition of bioorganic compounds, in addition to carbon atoms (C), which form the basis of any organic molecule, also includes hydrogen (H), oxygen (O), nitrogen (N), phosphorus (P) and sulfur (S). These bioelements (organogens) are concentrated in living organisms in quantities that are over 200 times higher than their content in inanimate objects. The noted elements make up over 99% of the elemental composition of biomolecules. 6


Bioorganic chemistry arose from the depths of organic chemistry and is based on its ideas and methods. In the history of development, organic chemistry has the following stages: empirical, analytical, structural and modern. The period from man's first acquaintance with organic substances to the end of the 18th century is considered empirical. The main result of this period is that people realized the importance of elemental analysis and the establishment of atomic and molecular weights. The theory of vitalism - life force (Berzelius). The analytical period continued until the 60s of the 19th century. It was marked by the fact that from the end of the first quarter of the 19th century a number of promising discoveries were made that dealt a crushing blow to the vitalistic theory. The first in this series was Berzelius's student, the German chemist Wöhler. He made a number of discoveries in 1824 - the synthesis of oxalic acid from cyanogen: (CN) 2 HOOC - COOH r. – synthesis of urea from ammonium cyanate: NH 4 CNO NH 2 – C – NH 2 O 8

In 1853, C. Gerard developed the “theory of types” and used it to classify organic compounds. According to Gerard, more complex organic compounds can be produced from the following basic four types substances: НННН type HYDROGEN НННН O type WATER H Cl type HYDROGEN CHLORIDE НННННН N type AMMONIA Since 1857, at the suggestion of F. A. Kekule, hydrocarbons began to be classified as methane type ННННННН С 9

Basic provisions of the theory of the structure of organic compounds (1861) 1) atoms in molecules are connected to each other by chemical bonds in accordance with their valence; 2) atoms in molecules of organic substances are connected to each other in a certain sequence, which determines the chemical structure (structure) of the molecule; 3) the properties of organic compounds depend not only on the number and nature of their constituent atoms, but also on the chemical structure of the molecules; 4) in organic molecules there is interaction between atoms, both bound to each other and unbound; 5) the chemical structure of a substance can be determined by studying its chemical transformations and, conversely, its properties can be characterized by the structure of a substance. 10

Basic principles of the theory of the structure of organic compounds (1861) Structural formula it is a picture of the sequence of bonds of atoms in a molecule. Gross formula – CH 4 O or CH 3 OH Structural formula Simplified structural formulas are sometimes called rational Molecular formula- the formula of an organic compound, which indicates the number of atoms of each element in the molecule. For example: C 5 H 12 - pentane, C 6 H 6 - gasoline, etc. eleven



Stages of development of bioorganic chemistry As a separate field of knowledge that combines the conceptual principles and methodology of organic chemistry on the one hand and molecular biochemistry and molecular pharmacology on the other hand, bioorganic chemistry was formed in the twentieth century based on developments in the chemistry of natural substances and biopolymers. Modern bioorganic chemistry has acquired fundamental significance thanks to the work of W. Stein, S. Moore, F. Sanger (analysis of amino acid composition and determination of the primary structure of peptides and proteins), L. Pauling and H. Astbury (clarification of the structure of the -helix and -structure and their significance in the implementation of the biological functions of protein molecules), E. Chargaff (deciphering the features of the nucleotide composition of nucleic acids), J. Watson, Fr. Crick, M. Wilkins, R. Franklin (establishing the patterns of the spatial structure of the DNA molecule), G. Corani ( chemical synthesis gene), etc. 14

Classification of organic compounds according to the structure of the carbon skeleton and the nature of the functional group The huge number of organic compounds prompted chemists to classify them. The classification of organic compounds is based on two classification characteristics: 1. Structure of the carbon skeleton 2. Nature of functional groups Classification according to the method of structure of the carbon skeleton: 1. Acyclic (alkanes, alkenes, alkynes, alkadienes); 2. Cyclic 2.1. Carbocyclic (alicyclic and aromatic) 2.2. Heterocyclic 15 Acyclic compounds are also called aliphatic. These include substances with an open carbon chain. Acyclic compounds are divided into saturated (or saturated) C n H 2n+2 (alkanes, paraffins) and unsaturated (unsaturated). The latter include alkenes C n H 2n, alkynes C n H 2n -2, alkadienes C n H 2n -2.

16 Cyclic compounds contain rings (cycles) within their molecules. If the cycles contain only carbon atoms, then such compounds are called carbocyclic. In turn, carbocyclic compounds are divided into alicyclic and aromatic. Alicyclic hydrocarbons (cycloalkanes) include cyclopropane and its homologues - cyclobutane, cyclopentane, cyclohexane, and so on. If the cyclic system, in addition to the hydrocarbon, also includes other elements, then such compounds are classified as heterocyclic.

Classification by the nature of the functional group A functional group is an atom or a group of atoms connected in a certain way, the presence of which in a molecule organic matter defines characteristic properties and its belonging to one or another class of compounds. Based on the number and homogeneity of functional groups, organic compounds are divided into mono-, poly- and heterofunctional. Substances with one functional group are called monofunctional; substances with several identical functional groups are called polyfunctional. Compounds containing several different functional groups are heterofunctional. It is important that compounds of the same class are combined into homologous series. A homologous series is a series of organic compounds with the same functional groups and the same structure; each representative of the homologous series differs from the previous one by a constant unit (CH 2), which is called the homologous difference. Members of a homologous series are called homologues. 17

Nomenclature systems in organic chemistry - trivial, rational and international (IUPAC) Chemical nomenclature a set of names of individual chemical substances, their groups and classes, as well as rules for compiling their names. Chemical nomenclature is a set of names of individual chemical substances, their groups and classes, as well as rules for compiling their names. The trivial (historical) nomenclature is associated with the process of obtaining substances (pyrogallol - a product of pyrolysis of gallic acid), the source of origin from which it was obtained (formic acid), etc. Trivial names of compounds are widely used in the chemistry of natural and heterocyclic compounds (citral, geraniol, thiophene, pyrrole, quinoline, etc.). Trivial (historical) nomenclature is associated with the process of obtaining substances (pyrogallol is a product of pyrolysis of gallic acid), the source of origin, from which was obtained (formic acid), etc. Trivial names of compounds are widely used in the chemistry of natural and heterocyclic compounds (citral, geraniol, thiophene, pyrrole, quinoline, etc.). Rational nomenclature is based on the principle of dividing organic compounds into homologous series. All substances in a certain homologous series are considered as derivatives of the simplest representative of a given series - the first or sometimes the second. In particular, for alkanes - methane, for alkenes - ethylene, etc. The rational nomenclature is based on the principle of dividing organic compounds into homologous series. All substances in a certain homologous series are considered as derivatives of the simplest representative of this series - the first or sometimes the second. In particular, for alkanes - methane, for alkenes - ethylene, etc. 18

International nomenclature (IUPAC). The rules of modern nomenclature were developed in 1957 at the 19th Congress of the International Union of Pure and Applied Chemistry (IUPAC). Radical functional nomenclature. These names are based on the name of the functional class (alcohol, ether, ketone, etc.), which is preceded by the names of hydrocarbon radicals, for example: alyl chloride, diethyl ether, dimethyl ketone, propyl alcohol, etc. Substitute nomenclature. Nomenclature rules. Parent structure - a structural fragment of a molecule (molecular skeleton) underlying the name of a compound, main carbon chain atoms for alicyclic compounds, for carbocyclic compounds - a cycle. 19

Chemical bond in organic molecules Chemical bond is the phenomenon of interaction between the outer electron shells (valence electrons of atoms) and atomic nuclei, which determines the existence of a molecule or crystal as a whole. As a rule, an atom, accepting or donating an electron or forming a common electron pair, tends to acquire a configuration of the outer electron shell similar to that of noble gases. The following types of chemical bonds are characteristic of organic compounds: - ionic bond - covalent bond - donor - acceptor bond - hydrogen bond. There are also some other types of chemical bonds (metallic, one-electron, two-electron three-center), but they are practically not found in organic compounds. 20



Types of bonds in organic compounds The most characteristic of organic compounds is a covalent bond. A covalent bond is the interaction of atoms, which is realized through the formation of a common electron pair. This type of bond is formed between atoms that have comparable electronegativity values. Electronegativity is a property of an atom that shows the ability to attract electrons to itself from other atoms. A covalent bond can be polar or non-polar. A non-polar covalent bond occurs between atoms with the same electronegativity value

Types of bonds in organic compounds A polar covalent bond is formed between atoms that have different electronegativity values. In this case, the bonded atoms acquire partial charges δ+δ+ δ-δ- A special subtype of covalent bond is the donor-acceptor bond. As in previous examples, this type of interaction is due to the presence of a common electron pair, but the latter is provided by one of the atoms forming the bond (donor) and accepted by another atom (acceptor) 24

Types of bonds in organic compounds Ionic bond is formed between atoms that differ greatly in electronegativity values. In this case, the electron of the less electronegative element (often a metal) is completely transferred to the more electronegative element. This electron transition causes the appearance of a positive charge on the less electronegative atom and a negative charge on the more electronegative one. Thus, two ions with opposite charges are formed, between which there is an electrovalent interaction. 25

Types of Bonds in Organic Compounds A hydrogen bond is an electrostatic interaction between a hydrogen atom, which is bonded in a highly polar manner, and electron pairs of oxygen, fluorine, nitrogen, sulfur and chlorine. This type of interaction is quite weak interaction. Hydrogen bonding can be intermolecular or intramolecular. Intermolecular hydrogen bond (interaction between two molecules ethyl alcohol) Intramolecular hydrogen bond in salicylic aldehyde 26

Chemical bonding in organic molecules Modern theory chemical bond is based on the quantum mechanical model of a molecule as a system consisting of electrons and atomic nuclei. The cornerstone concept of quantum mechanical theory is the atomic orbital. An atomic orbital is a part of space in which the probability of finding electrons is maximum. Bonding can thus be viewed as the interaction (“overlap”) of orbitals that each carry one electron with opposite spins. 27

Hybridization of atomic orbitals According to quantum mechanical theory, the number of covalent bonds formed by an atom is determined by the number of one-electron atomic orbitals (the number of unpaired electrons). The carbon atom has only two unpaired electrons in its ground state, but possible transition electron from 2s to 2 pz makes it possible to form four covalent bonds. The state of a carbon atom in which it has four unpaired electrons is called “excited.” Despite the fact that carbon orbitals are unequal, it is known that the formation of four equivalent bonds is possible due to the hybridization of atomic orbitals. Hybridization is a phenomenon in which the same number of orbitals of the same shape and number are formed from several orbitals of different shapes and similar in energy. 28



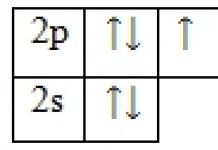
Hybrid states of the carbon atom in organic molecules FIRST HYBRID STATE The C atom is in a state of sp 3 hybridization, forms four σ bonds, forms four hybrid orbitals, which are arranged in the shape of a tetrahedron (bond angle) σ bond 31

Hybrid states of the carbon atom in organic molecules SECOND HYBRID STATE The C atom is in a state of sp 2 hybridization, forms three σ-bonds, forms three hybrid orbitals, which are arranged in the shape of a flat triangle (bond angle 120) σ-bonds π-bond 32

Hybrid states of the carbon atom in organic molecules THIRD HYBRID STATE The C atom is in a state of sp-hybridization, forms two σ-bonds, forms two hybrid orbitals, which are arranged in a line (bond angle 180) σ-bonds π-bonds 33




Characteristics of chemical bonds POLING scale: F-4.0; O – 3.5; Cl – 3.0; N – 3.0; Br – 2.8; S – 2.5; C-2.5; H-2.1. difference 1.7
Characteristics of chemical bonds Bond polarizability is a shift in electron density under the influence of external factors. Bond polarizability is the degree of electron mobility. As the atomic radius increases, the polarizability of electrons increases. Therefore, the polarizability of the Carbon - halogen bond increases as follows: C-F 
Electronic effects. Mutual influence of atoms in a molecule 39 According to modern theoretical concepts, the reactivity of organic molecules is predetermined by the displacement and mobility of electron clouds that form a covalent bond. In organic chemistry, two types of electron displacements are distinguished: a) electronic displacements occurring in the -bond system, b) electronic displacements transmitted by the -bond system. In the first case, the so-called inductive effect takes place, in the second - a mesomeric effect. The inductive effect is a redistribution of electron density (polarization) resulting from the difference in electronegativity between the atoms of a molecule in a system of -bonds. Due to the insignificant polarizability of the -bonds, the inductive effect quickly fades away and after 3-4 bonds it almost does not appear.

Electronic effects. Mutual influence of atoms in a molecule 40 The concept of the inductive effect was introduced by K. Ingold, and he also introduced the following designations: –I-effect in the case of a decrease in electron density by a substituent +I-effect in the case of an increase in electron density by a substituent A positive inductive effect is exhibited by alkyl radicals (CH 3, C 2 H 5 - etc.). All other substituents bonded to the carbon atom exhibit a negative inductive effect.

Electronic effects. Mutual influence of atoms in a molecule 41 The mesomeric effect is the redistribution of electron density along a conjugated system. Conjugated systems include molecules of organic compounds in which double and single bonds alternate or when an atom with a lone pair of electrons in the p-orbital is located next to the double bond. In the first case, - conjugation takes place, and in the second case, p, -conjugation takes place. Coupled systems come in open and closed circuit types. Examples of such compounds are 1,3-butadiene and gasoline. In the molecules of these compounds, the carbon atoms are in a state of sp 2 hybridization and, due to non-hybrid p-orbitals, form -bonds that mutually overlap each other and form a single electron cloud, that is, conjugation takes place.

Electronic effects. Mutual influence of atoms in a molecule 42 There are two types of mesomeric effect - positive mesomeric effect (+M) and negative mesomeric effect (-M). A positive mesomeric effect is exhibited by substituents that provide p-electrons to the conjugated system. These include: -O, -S -NH 2, -OH, -OR, Hal (halogens) and other substituents that have a negative charge or a lone pair of electrons. The negative mesomeric effect is characteristic of substituents that absorb electron density from the conjugated system. These include substituents that have multiple bonds between atoms with different electronegativity: - N0 2 ; -SO 3 H; >C=O; -COON and others. The mesomeric effect is graphically reflected by a bent arrow, which shows the direction of electron displacement. Unlike the induction effect, the mesomeric effect does not go out. It is transmitted completely throughout the system, regardless of the length of the interfacing chain. C=O; -COON and others. The mesomeric effect is graphically reflected by a bent arrow, which shows the direction of electron displacement. Unlike the induction effect, the mesomeric effect does not go out. It is transmitted completely throughout the system, regardless of the length of the interfacing chain."> 
Types of chemical reactions 43 A chemical reaction can be considered as the interaction of a reagent and substrate. Depending on the method of breaking and forming a chemical bond in molecules, organic reactions divided into: a) homolytic b) heterolytic c) molecular Homolytic or free radical reactions are caused by homolytic cleavage of the bond, when each atom has one electron left, that is, radicals are formed. Homolytic rupture occurs when high temperatures, the action of a quantum of light or catalysis.

Heterolytic or ionic reactions proceed in such a way that a pair of bonding electrons remains near one of the atoms and ions are formed. A particle with an electron pair is called nucleophilic and has a negative charge (-). A particle without an electron pair is called electrophilic and has a positive charge (+). 44 Types of chemical reactions

Mechanism of a chemical reaction 45 The mechanism of a reaction is the set of elementary (simple) stages that make up a given reaction. The reaction mechanism most often includes the following stages: activation of the reagent with the formation of an electrophile, nucleophile or free radical. To activate a reagent, a catalyst is usually needed. In the second stage, the activated reagent interacts with the substrate. In this case, intermediate particles (intermediates) are formed. The latter include -complexes, -complexes (carbocations), carbanions, and new free radicals. At the final stage, the addition or elimination of a particle to (from) the intermediate formed in the second stage takes place with the formation of the final reaction product. If a reagent generates a nucleophile upon activation, then these are nucleophilic reactions. They are marked with the letter N - (in the index). In the case where the reagent generates an electrophile, the reactions are classified as electrophilic (E). The same can be said about free radical reactions (R).

Nucleophiles are reagents that have a negative charge or an atom enriched in electron density: 1) anions: OH -, CN -, RO -, RS -, Hal - and other anions; 2) neutral molecules with lone pairs of electrons: NH 3, NH 2 R, H 2 O, ROH and others; 3) molecules with excess electron density (having - bonds). Electrophiles are reagents that have a positive charge or an atom depleted in electron density: 1) cations: H + (proton), HSO 3 + (hydrogen sulfonium ion), NO 2 + (nitronium ion), NO (nitrosonium ion) and other cations; 2) neutral molecules with a vacant orbital: AlCl 3, FeBr 3, SnCl 4, BF 4 (Lewis acids), SO 3; 3) molecules with depleted electron density on the atom. 46



49

50

51

52


Hello! Many medical students are now studying bioorganic chemistry, also known as biochemistry.
In some universities this subject ends with a test, in others – with an exam. Sometimes it happens that a test at one university is comparable in difficulty to an exam at another.
At my university, bioorganic chemistry was taken as an exam during the summer session at the very end of the first year. It must be said that BOC is one of those subjects that is terrifying at first and can inspire the thought “this is impossible to pass.” This is especially true, of course, for people with a weak foundation in organic chemistry (and, oddly enough, there are quite a few of them in medical universities).
Programs for studying bioorganic chemistry at different universities can vary greatly, and teaching methods can vary even more.
However, the requirements for students are approximately the same everywhere. To put it very simply, in order to pass bioorganic chemistry with a 5, you must know the names, properties, structural features and typical reactions of a number of organic substances.
Our teacher, a respected professor, presented the material as if each student was the best organic chemistry student in school (and bioorganic chemistry is essentially a complicated course in school organic chemistry). He was probably right in his approach, everyone should strive to reach the top and try to be the best. However, this led to the fact that some students, who did not partially understand the material in the first 2-3 classes, stopped understanding everything altogether closer to the middle of the semester.
I decided to write this material largely because I was just such a student. At school I really loved inorganic chemistry, but I always struggled with organics. Even when I was preparing for the Unified State Exam, I chose the strategy of strengthening all my knowledge in inorganics, while at the same time consolidating only the base of organics. By the way, this almost backfired on me in terms of entrance points, but that’s another story.
It was not in vain that I said about the teaching methodology, because ours was also very unusual. Right away, almost in the first class, we were shown the manuals according to which we had to take tests and then an exam.
Bioorganic chemistry - tests and exam
Our entire course was divided into 4 major topics, each of which ended with a test lesson. We already had questions for each of the four tests from the first couple. They were, of course, frightening, but at the same time they served as a kind of map along which to move.
The first test was quite basic. It was devoted mainly to nomenclature, trivial (everyday) and international names, and, of course, classification of substances. Also, in one form or another, the signs of aromaticity were touched upon.
The second test after the first seemed much more difficult. There it was necessary to describe the properties and reactions of substances such as ketones, aldehydes, alcohols, and carboxylic acids. For example, one of the most typical reactions of aldehydes is the reaction silver mirror. Quite a beautiful sight. If you add Tollens’ reagent, that is, OH, to any aldehyde, then on the wall of the test tube you will see a precipitate that resembles a mirror, this is what it looks like: 
The third test compared to the second did not seem so formidable. Everyone is already accustomed to writing reactions and remembering properties according to classifications. In the third test we talked about compounds with two functional groups - aminophenols, amino alcohols, oxoacids and others. Also, each ticket contained at least one ticket about carbohydrates.
The fourth test in bioorganic chemistry was almost entirely devoted to proteins, amino acids and peptide bonds. A special highlight were the questions that required collecting RNA and DNA.
By the way, this is exactly what an amino acid looks like - you can see the amino group (it is tinted yellow in this picture) and the carboxylic acid group (it is lilac). It was substances of this class that we had to deal with in the fourth test. 
Each test was taken at the blackboard - the student must, without prompting, describe and explain all the necessary properties in the form of reactions. For example, if you are taking the second test, you have the properties of alcohols on your ticket. The teacher tells you to take propanol. You write the formula of propanol and 4-5 typical reactions to illustrate its properties. There could also be something exotic, like sulfur-containing compounds. An error even in the index of one reaction product often sent me further to study this material until the next attempt (which was a week later). Scary? Harsh? Certainly!
However, this approach has a very pleasant side effect. It was hard during regular seminar classes. Many took the tests 5-6 times. But the exam was very easy, because each ticket contained 4 questions. Exactly, one from each already learned and solved test.
Therefore, I will not even describe the intricacies of preparing for the exam in bioorganic chemistry. In our case, all preparation came down to how we prepared for the tests themselves. I confidently passed each of the four tests - before the exam, just look through your own drafts, write down the most basic reactions and everything will be restored right away. The fact is that organic chemistry is a very logical science. What you need to remember is not the huge strings of reactions, but the mechanisms themselves.
Yes, I note that this does not work with all items. You won't be able to pass the formidable anatomy by simply reading your notes the day before. A number of other items also have their own characteristics. Even if in your medical university Bioorganic chemistry is taught differently, you may need to adjust your preparation and do it a little differently than I did. In any case, good luck to you, understand and love science!
Chemistry- the science of the structure, properties of substances, their transformations and accompanying phenomena.
Tasks:
1. Study of the structure of matter, development of the theory of the structure and properties of molecules and materials. It is important to establish a connection between the structure and various properties of substances and, on this basis, to construct theories of the reactivity of a substance, the kinetics and mechanism of chemical reactions and catalytic phenomena.
2. Implementation of targeted synthesis of new substances with specified properties. Here it is also important to find new reactions and catalysts for more efficient synthesis of already known and industrially important compounds.
3. The traditional task of chemistry has acquired special significance. It is associated both with an increase in the number of chemical objects and properties being studied, and with the need to determine and reduce the consequences of human impact on nature.
Chemistry is a general theoretical discipline. It is designed to give students modern scientific presentation about matter as one of the types of moving matter, about the ways, mechanisms and methods of converting some substances into others. Knowledge of basic chemical laws, mastery of chemical calculation techniques, understanding of the opportunities provided by chemistry with the help of other specialists working in its individual and narrow fields significantly speeds up obtaining the desired result in various fields of engineering and scientific activity.
The chemical industry is one of the most important industries in our country. The chemical compounds, various compositions and materials it produces are used everywhere: in mechanical engineering, metallurgy, agriculture, construction, electrical and electronic industry, communications, transport, space technology, medicine, everyday life, etc. The main directions of development of the modern chemical industry are: the production of new compounds and materials and increasing the efficiency of existing production.
At a medical school, students study general, bioorganic, biological chemistry, as well as clinical biochemistry. Students' knowledge of the complex of chemical sciences in their continuity and interconnection provides greater opportunity, greater scope for research and practical use of various phenomena, properties and patterns, and contributes to personal development.
Specific features studying chemical disciplines at a medical university are:
· interdependence between the goals of chemical and medical education;
· universality and fundamentality of these courses;
· the peculiarity of constructing their content depending on the nature and general goals of the doctor’s training and his specialization;
· unity of studying chemical objects at micro- and macro-levels with the disclosure of their different forms chemical organization How unified system and the various functions it exhibits (chemical, biological, biochemical, physiological, etc.) depending on their nature, environment and conditions;
· dependence on the connection of chemical knowledge and skills with reality and practice, including medical practice, in the system “society - nature - production - man”, due to the unlimited possibilities of chemistry in the creation of synthetic materials and their importance in medicine, the development of nanochemistry, as well as in solving environmental and many other global problems humanity.
1. The relationship between metabolic processes and energy in the body
Life processes on Earth are determined to a large extent by the accumulation of solar energy in nutrients - proteins, fats, carbohydrates and the subsequent transformations of these substances in living organisms with the release of energy. The understanding of the relationship between chemical transformations and energy processes in the body was realized especially clearly after works by A. Lavoisier (1743-1794) and P. Laplace (1749-1827). They showed by direct calorimetric measurements that the energy released during life is determined by the oxidation of food by air oxygen inhaled by animals.
Metabolism and energy - a set of processes of transformation of substances and energy occurring in living organisms, and the exchange of substances and energy between the body and environment. Metabolism of substances and energy is the basis of the life of organisms and is one of the most important specific characteristics of living matter, distinguishing living from non-living. Metabolism, or metabolism, which is ensured by highly complex regulation at different levels, involves many enzyme systems. During the metabolic process, substances entering the body are converted into tissues’ own substances and into final products excreted from the body. During these transformations, energy is released and absorbed.
With the development in the XIX-XX centuries. thermodynamics - the science of the interconversion of heat and energy - it became possible to quantitatively calculate the transformation of energy in biochemical reactions and predict their direction.
Energy exchange can be carried out by transferring heat or doing work. However, living organisms are not in equilibrium with their environment and therefore can be called non-equilibrium open systems. However, when observed over a certain period of time, there are no visible changes in the chemical composition of the body. But that doesn't mean that chemical substances, making up the body, do not undergo any transformations. On the contrary, they are constantly and quite intensively renewed, as can be judged by the rate at which stable isotopes and radionuclides introduced into the cell as part of simpler precursor substances are incorporated into complex substances of the body.
There is one thing between metabolism and energy metabolism fundamental difference. The earth does not lose or gain any appreciable amount of matter. Matter in the biosphere is exchanged in a closed cycle, etc. used repeatedly. Energy exchange is carried out differently. It does not circulate in a closed cycle, but is partially dispersed into external space. Therefore, to maintain life on Earth, a constant flow of energy from the Sun is necessary. For 1 year in the process of photosynthesis on globe absorbed around 10 21 feces solar energy. Although it represents only 0.02% of the total energy of the Sun, it is immeasurably more than the energy used by all man-made machines. The amount of substance participating in the circulation is equally large.
2. Chemical thermodynamics as theoretical basis bioenergy. Subject and methods of chemical thermodynamics
Chemical thermodynamics studies the transitions of chemical energy into other forms - thermal, electrical, etc., establishes the quantitative laws of these transitions, as well as the direction and limits of the spontaneous occurrence of chemical reactions under given conditions.
The thermodynamic method is based on a number of strict concepts: “system”, “state of the system”, “ internal energy systems", "system state function".
Object studying in thermodynamics is a system
The same system can be in different states. Each state of the system is characterized by a certain set of values of thermodynamic parameters. Thermodynamic parameters include temperature, pressure, density, concentration, etc. A change in at least one thermodynamic parameter leads to a change in the state of the system as a whole. The thermodynamic state of a system is called equilibrium if it is characterized by constancy of thermodynamic parameters at all points of the system and does not change spontaneously (without the expenditure of work).
Chemical thermodynamics studies a system in two equilibrium states (final and initial) and on this basis determines the possibility (or impossibility) of a spontaneous process under given conditions in a specified direction.
Thermodynamics studies mutual transformations various types energies associated with the transfer of energy between bodies in the form of heat and work. Thermodynamics is based on two basic laws, called the first and second laws of thermodynamics. Subject of study in thermodynamics is energy and the laws of mutual transformations of energy forms during chemical reactions, processes of dissolution, evaporation, crystallization.
Chemical thermodynamics is a branch of physical chemistry that studies the processes of interaction of substances using thermodynamic methods.
The main directions of chemical thermodynamics are:
Classical chemical thermodynamics, which studies thermodynamic equilibrium in general.
Thermochemistry, which studies the thermal effects accompanying chemical reactions.
The theory of solutions, which models the thermodynamic properties of a substance based on ideas about the molecular structure and data on intermolecular interaction.
Chemical thermodynamics is closely related to such branches of chemistry as analytical chemistry; electrochemistry; colloid chemistry; adsorption and chromatography.
The development of chemical thermodynamics proceeded simultaneously in two ways: thermochemical and thermodynamic.
The emergence of thermochemistry as an independent science should be considered the discovery by Herman Ivanovich Hess, a professor at St. Petersburg University, of the relationship between the thermal effects of chemical reactions -- Hess's laws.
3. Thermodynamic systems: isolated, closed, open, homogeneous, heterogeneous. The concept of phase.
System- this is a collection of interacting substances, mentally or actually isolated from the environment (test tube, autoclave).
Chemical thermodynamics considers transitions from one state to another, while some may change or remain constant. options:
· isobaric– at constant pressure;
· isochoric– at constant volume;
· isothermal– at constant temperature;
· isobaric - isothermal– at constant pressure and temperature, etc.
The thermodynamic properties of a system can be expressed using several system state functions, called characteristic functions: internal energyU , enthalpy H , entropy S , Gibbs energy G , Helmholtz energy F . Characteristic functions have one feature: they do not depend on the method (path) of achieving a given state of the system. Their value is determined by the parameters of the system (pressure, temperature, etc.) and depends on the amount or mass of the substance, so it is customary to refer them to one mole of the substance.
According to the method of transferring energy, matter and information between the system under consideration and the environment, thermodynamic systems are classified:
1. Closed (isolated) system- this is a system in which there is no exchange of energy, matter (including radiation), or information with external bodies.
2. Closed system- a system in which there is an exchange only with energy.
3. Adiabatically isolated system - This is a system in which there is an exchange of energy only in the form of heat.
4. Open system is a system that exchanges energy, matter, and information.
System classification:
1) if heat and mass transfer are possible: insulated, closed, open. An isolated system does not exchange either matter or energy with the environment. A closed system exchanges energy with the environment, but does not exchange matter. An open system exchanges both matter and energy with its environment. The concept of an isolated system is used in physical chemistry as a theoretical one.
2) by internal structure and properties: homogeneous and heterogeneous. A system is called homogeneous, inside which there are no surfaces dividing the system into parts that differ in properties or chemical composition. Examples of homogeneous systems are aqueous solutions of acids, bases, and salts; mixtures of gases; individual pure substances. Heterogeneous systems contain natural surfaces within them. Examples of heterogeneous systems are systems consisting of different state of aggregation substances: metal and acid, gas and solid, two liquids insoluble in each other.
Phase- this is a homogeneous part of a heterogeneous system, having the same composition, physical and chemical properties, separated from other parts of the system by a surface, upon passing through which the properties of the system change abruptly. The phases are solid, liquid and gaseous. A homogeneous system always consists of one phase, a heterogeneous one - of several. Based on the number of phases, systems are classified into single-phase, two-phase, three-phase, etc.
5.The first law of thermodynamics. Internal energy. Isobaric and isochoric thermal effects .
First law of thermodynamics- one of the three basic laws of thermodynamics, represents the law of conservation of energy for thermodynamic systems.
The first law of thermodynamics was formulated in the middle of the 19th century as a result of the work of the German scientist J. R. Mayer, the English physicist J. P. Joule and the German physicist G. Helmholtz.
According to the first law of thermodynamics, a thermodynamic system can undergo work only due to its internal energy or any external energy sources .
The first law of thermodynamics is often formulated as the impossibility of the existence of a perpetual motion machine of the first kind, which would do work without drawing energy from any source. A process occurring at a constant temperature is called isothermal, at constant pressure - isobaric, at constant volume – isochoric. If during a process the system is isolated from the external environment in such a way that heat exchange with the environment is excluded, the process is called adiabatic.
Internal energy of the system. When a system transitions from one state to another, some of its properties change, in particular internal energy U.
The internal energy of a system is its total energy, which consists of the kinetic and potential energies of molecules, atoms, atomic nuclei and electrons. Internal energy includes the energy of translational, rotational and oscillatory movements, as well as potential energy due to the forces of attraction and repulsion acting between molecules, atoms and intra-atomic particles. It does not include the potential energy of the system’s position in space and the kinetic energy of the system’s motion as a whole.
Internal energy is a thermodynamic function of the state of the system. This means that whenever the system finds itself in a given state, its internal energy takes on a certain value inherent in this state.
∆U = U 2 - U 1
where U 1 and U 2 are the internal energy of the system V final and initial states, respectively.
First law of thermodynamics. If the system exchanges thermal energy Q and mechanical energy(work) A, and at the same time transitions from state 1 to state 2, the amount of energy that is released or absorbed by the system of forms of heat Q or work A is equal to the total energy of the system during the transition from one state to another and is recorded.
Bioorganic chemistry. Tyukavkina N.A., Baukov Yu.I.

3rd ed., revised. and additional - M.: 2004 - 544 p.
The main feature of the textbook is the combination of the medical focus of this chemical course, required for medical students, with its high, fundamental scientific level. The textbook includes basic material on the structure and reactivity of organic compounds, including biopolymers, which are structural components of the cell, as well as the main metabolites and low-molecular bioregulators. In the third edition (2nd - 1991), special attention is paid to compounds and reactions that have analogies in a living organism, the emphasis on highlighting the biological role of important classes of compounds is increased, and the range of modern information of an ecological and toxicological nature is expanded. For university students studying in specialties 040100 General Medicine, 040200 Pediatrics, 040300 Medical and Preventive Care, 040400 Dentistry.
Format: pdf
Size: 15 MB
Watch, download:drive.google
CONTENT
Preface................................... 7
Introduction........................ 9
Part I
BASICS OF STRUCTURE AND REACTIVITY OF ORGANIC COMPOUNDS
Chapter 1. General characteristics of organic compounds 16
1.1. Classification. "................ 16
1.2. .Nomenclature............... 20
1.2.1. Substitute nomenclature........... 23
1.2.2. Radical functional nomenclature........ 28
Chapter 2. Chemical bonding and mutual influence of atoms in organic
connections......................... 29
2.1. Electronic structure of organogen elements...... 29
2.1.1. Atomic orbitals................ 29
2.1.2. Orbital hybridization......................... 30
2.2. Covalent bonds............... 33
2.2.1. a- and l-Connections......................... 34
2.2.2. Donor-acceptor bonds............ 38
2.2.3. Hydrogen bonds............... 39
2.3. Conjugation and aromaticity............ 40
2.3.1. Open circuit systems... ,..... 41
2.3.2. Closed-loop systems........ 45
2.3.3. Electronic effects......................... 49
Chapter 3. Fundamentals of the structure of organic compounds....... 51
3.1. Chemical structure And structural isomerism...... 52
3.2. Spatial structure and stereoisomerism...... 54
3.2.1. Configuration................... 55
3.2.2. Conformation................... 57
3.2.3. Elements of symmetry of molecules............ 68
3.2.4. Eianthiomeria......... 72
3.2.5. Diastereomerism............
3.2.6. Racemates................... 80
3.3. Enantiotopy, diastereotopy. . ......... 82
Chapter 4 General characteristics of reactions of organic compounds 88
4.1. The concept of the reaction mechanism..... 88
3
11.2. Primary structure of peptides and proteins........ 344
11.2.1. Composition and amino acid sequence...... 345
11.2.2. Structure and synthesis of peptides............ 351
11.3. Spatial structure of polypeptides and proteins.... 361
Chapter 12. Carbohydrates.................................... 377
12.1. Monosaccharides................... 378
12.1.1. Structure and stereoisomerism................... 378
12.1.2. Tautomerism..............." . 388
12.1.3. Conformations................... 389
12.1.4. Derivatives of monosaccharides............ 391
12.1.5. Chemical properties............... 395
12.2. Disaccharides................... 407
12.3. Polysaccharides................... 413
12.3.1. Homopolysaccharides............... 414
12.3.2. Heteropolysaccharides............... 420
Chapter 13. Nucleotides and nucleic acids.........431
13.1. Nucleosides and nucleotides.............. 431
13.2. Structure of nucleic acids........... 441
13.3 Nucleoside polyphosphates. Nicotinamide nucleotides..... 448
Chapter 14. Lipids and low-molecular bioregulators...... 457
14.1. Saponifiable lipids........................ 458
14.1.1. Higher fatty acids - structural components of saponifiable lipids 458
14.1.2. Simple lipids................ 461
14.1.3. Complex lipids................ 462
14.1.4. Some properties of saponified lipids and their structural components 467
14.2. Unsaponifiable lipids 472
14.2.1. Terpenes......... ...... 473
14.2.2. Low molecular weight bioregulators of lipid nature. . . 477
14.2.3. Steroids................... 483
14.2.4. Biosynthesis of terpenes and steroids........... 492
Chapter 15. Methods for studying organic compounds...... 495
15.1. Chromatography................... 496
15.2. Analysis of organic compounds. . ........ 500
15.3. Spectral methods................... 501
15.3.1. Electron spectroscopy............... 501
15.3.2. Infrared spectroscopy............ 504
15.3.3. Nuclear magnetic resonance spectroscopy...... 506
15.3.4. Electron paramagnetic resonance......... 509
15.3.5. Mass spectrometry............... 510
Preface
Over the centuries-old history of the development of natural science, a close relationship has been established between medicine and chemistry. The current deep interpenetration of these sciences leads to the emergence of new scientific directions that study the molecular nature of individual physiological processes, the molecular basis of the pathogenesis of diseases, molecular aspects of pharmacology, etc. The need to understand life processes at the molecular level is understandable, “because living cell- a real kingdom of large and small molecules, continuously interacting, arising and disappearing”*.
Bioorganic chemistry studies biologically significant substances and can serve as a “molecular tool” for the versatile study of cell components.
Bioorganic chemistry plays an important role in the development of modern fields of medicine and is an integral part of the natural science education of a doctor.
The progress of medical science and improvement of healthcare are associated with deep fundamental training of specialists. The relevance of this approach is largely determined by the transformation of medicine into a large industry social sphere, which focuses on problems of ecology, toxicology, biotechnology, etc.
Due to the lack of curriculum medical universities general course organic chemistry in this textbook a certain place is given to the basics of organic chemistry, necessary for the assimilation of bioorganic chemistry. In preparing the third edition (2nd - 1992), the textbook material was revised and brought even closer to the tasks of perceiving medical knowledge. The range of compounds and reactions that have analogies in living organisms has been expanded. More attention is paid to environmental and toxicological information. Elements of a purely chemical nature, which are not of fundamental importance for medical education, have undergone some reduction, in particular, methods for obtaining organic compounds, the properties of a number of individual representatives, etc. At the same time, sections have been expanded to include material on the relationship between the structure of organic substances and their biological acting as the molecular basis for the action of drugs. The structure of the textbook has been improved, it has been placed in separate sections chemical material, which has special medical and biological significance.
The authors express their sincere gratitude to Professors S. E. Zurabyan, I. Yu. Belavin, I. A. Selivanova, as well as all colleagues for useful tips and assistance in preparing the manuscript for republication.