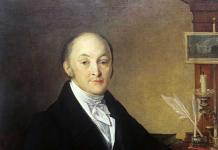Silicon (Si) - stands in period 3, group IV of the main subgroup periodic system. Physical properties: silicon exists in two modifications: amorphous and crystalline. Amorphous silicon is a brown powder with a density of 2.33 g/cm3, which dissolves in metal melts. Crystalline silicon is dark gray crystals with a steel luster, hard and brittle, with a density of 2.4 g/cm3. Silicon consists of three isotopes: Si (28), Si (29), Si (30).
Chemical properties: electronic configuration: 1s22s22p63 s23p2 . Silicon is a non-metal. At the external energy level, silicon has 4 electrons, which determines its oxidation states: +4, -4, -2. Valence - 2, 4. Amorphous silicon has a greater reactivity than crystalline. Under normal conditions, it interacts with fluorine: Si + 2F2 = SiF4. At 1000 °C, Si reacts with non-metals: with CL2, N2, C, S.
Of the acids, silicon interacts only with a mixture of nitric and hydrofluoric acids:
With respect to metals, it behaves differently: it dissolves well in molten Zn, Al, Sn, Pb, but does not react with them; with other melts of metals - with Mg, Cu, Fe, silicon interacts with the formation of silicides: Si + 2Mg = Mg2Si. Silicon burns in oxygen: Si + O2 = SiO2 (sand).
Silicon dioxide or silica- stable connection Si, is widely distributed in nature. It reacts with its fusion with alkalis, basic oxides, forming salts of silicic acid - silicates. Receipt: in industry, pure silicon is obtained by reduction of silicon dioxide with coke in electric furnaces: SiO2 + 2С = Si + 2СO?.
In the laboratory, silicon is obtained by calcining white sand with magnesium or aluminum:
SiO2 + 2Mg = 2MgO + Si.
3SiO2 + 4Al = Al2O3 + 3Si.
Silicon forms acids: H2 SiO3 - meta-silicic acid; H2 Si2O5 is two metasilicic acid.
Finding in nature: quartz mineral - SiO2. Quartz crystals have the shape of a hexagonal prism, colorless and transparent, called rock crystal. Amethyst - rock crystal, dyed purple with impurities; smoky topaz is painted brownish; agate and jasper are crystalline varieties of quartz. Amorphous silica is less common and exists in the form of the mineral opal, SiO2 nH2O. Diatomaceous earth, tripolite or kieselguhr (diatomaceous earth) are earthy forms of amorphous silicon.
42. The concept of colloidal solutions
Colloidal solutions– highly dispersed two-phase systems consisting of a dispersion medium and a dispersed phase. Particle sizes are intermediate between true solutions, suspensions and emulsions. At colloidal particles molecular or ionic composition.
There are three types of internal structure of primary particles.
1. Suspensoids (or irreversible colloids)– heterogeneous systems, the properties of which can be determined by a developed interfacial surface. Compared to suspensions, they are more highly dispersed. They cannot exist for a long time without a dispersion stabilizer. They are called irreversible colloids due to the fact that their precipitation after evaporation again does not form sols. Their concentration is low - 0.1%. They differ slightly from the viscosity of the dispersed medium.
Suspensoids can be obtained:
1) dispersion methods (grinding large bodies);
2) condensation methods (obtaining insoluble compounds by means of exchange reactions, hydrolysis, etc.).
The spontaneous decrease in dispersion in suspensoids depends on the free surface energy. To obtain a long-lasting suspension, conditions are necessary for its stabilization.
Stable disperse systems:
1) dispersion medium;
2) dispersed phase;
3) stabilizer of the dispersed system.
The stabilizer can be ionic, molecular, but most often high-molecular.
Protective colloids- macromolecular compounds that are added for stabilization (proteins, peptides, polyvinyl alcohol, etc.).
2. Associative (or micellar colloids) - semi-colloids arising at a sufficient concentration of molecules consisting of hydrocarbon radicals (amphiphilic molecules) of low molecular weight substances during their association into aggregates of molecules (micelles). Micelles are formed in aqueous solutions of detergents (soaps), organic dyes.
3. Molecular colloids (reversible or lyophilic colloids) - natural and synthetic high molecular weight substances. Their molecules have the size of colloidal particles (macromolecules).
Dilute solutions of colloids of macromolecular compounds are homogeneous solutions. When strongly diluted, these solutions obey the laws of dilute solutions.
Non-polar macromolecules dissolve in hydrocarbons, polar ones - in polar solvents.
Reversible colloids- substances, the dry residue of which, when a new portion of the solvent is added, again goes into solution.
Silicon was discovered and obtained in 1823 by the Swedish chemist Jens Jakob Berzelius.
The second most abundant element in the earth's crust after oxygen (27.6% by mass). Found in compounds.
|
The structure of the silicon atom in the ground state 1s 2 2s 2 2p 6 3s 2 3p 2 |
The structure of the silicon atom in an excited state 1s 2 2s 2 2p 6 3s 1 3p 3 Oxidation states: +4, -4. |
Allotropy of silicon
Amorphous and crystalline silicon are known.
polycrystalline silicon
Crystal - dark gray substance with a metallic sheen, high hardness, brittle, semiconductor; ρ \u003d 2.33 g / cm 3, t ° pl. =1415°C; t°boiling = 2680°C.
It has a diamond-like structure and forms strong covalent bonds. Inert.
Amorphous - brown powder, hygroscopic, diamond-like structure, ρ = 2 g/cm 3 , more reactive.
Getting silicon
1) Industry – heating coal with sand:
2C + SiO 2 t ˚ → Si + 2CO
2) Laboratory – heating sand with magnesium:
2Mg + SiO 2 t ˚ → Si + 2MgO Experience
Chemical properties
Typical non-metal, inert.
As a restorer:
1) With oxygen
Si 0 + O 2 t ˚ → Si +4 O 2
2) With fluorine (without heating)
Si 0 + 2F 2 → SiF 4
3) With carbon
Si 0 + C t ˚ → Si +4 C
(SiC - carborundum - hard; used for pointing and grinding)
4) Does not interact with hydrogen.
Silane (SiH 4) is obtained by decomposition of metal silicides with acid:
Mg 2 Si + 2H 2 SO 4 → SiH 4 + 2MgSO 4
5) Does not react with acids (Tonly with hydrofluoric acid Si+4 HF= SiF 4 +2 H 2 )
It dissolves only in a mixture of nitric and hydrofluoric acids:
3Si + 4HNO 3 + 18HF →3H 2 + 4NO + 8H 2 O
6) With alkalis (when heated):
As an oxidizing agent:
7) With metals (silicides are formed):
Si 0 + 2Mg t ˚ →Mg 2 Si -4
Silicon is widely used in electronics as a semiconductor. Silicon additions to alloys increase their corrosion resistance. Silicates, aluminosilicates and silica are the main raw materials for the production of glass and ceramics, as well as for the construction industry.Silicon in engineering
The use of silicon and its compounds
Silane - SiH 4
Physical properties: Colorless gas, poisonous, t°pl. = -185°C, bp = -112°C.
Obtaining silicic acid
The action of strong acids on silicates - Na 2 SiO 3 + 2HCl → 2NaCl + H 2 SiO 3 ↓
Chemical properties:
When heated, it decomposes: H 2 SiO 3 t ˚ → H 2 O + SiO 2
Salts of silicic acid - silicates.
1) with acids
Na 2 SiO 3 + H 2 O + CO 2 \u003d Na 2 CO 3 + H 2 SiO 3

2) with salts
Na 2 SiO 3 + CaCl 2 \u003d 2NaCl + CaSiO 3 ↓
3) Silicates, which are part of minerals, in natural conditions are destroyed under the action of water and carbon monoxide (IV) - weathering of rocks:
(K 2 O Al 2 O 3 6SiO 2) (feldspar) + CO 2 + 2H 2 O → (Al 2 O 3 2SiO 2 2H 2 O) (kaolinite (clay)) + 4SiO 2 (silica (sand)) + K2CO3
The use of silicon compounds

Natural silicon compounds - sand (SiO 2) and silicates are used for the production of ceramics, glass and cement.

|
Ceramics |
|
|
Porcelain= kaolin + clay + quartz + feldspar. The birthplace of porcelain is China, where porcelain has been known since 220. In 1746, the production of porcelain was established in Russia
|
Faience - from the name of the Italian city of Faenza. Where ceramic handicrafts were developed in the 14th and 15th centuries. Faience - differs from porcelain in a high content of clay (85%), a lower firing temperature. |
The content of the article
SILICON, Si (silicium), chemical element IVA subgroups (C, Si, Ge, Sn and Pb) of the Periodic Table of Elements, non-metal. Silicon in free form was isolated in 1811 by J. Gay-Lussac and L. Tenard by passing vapors of silicon fluoride over metallic potassium, but it was not described by them as an element. The Swedish chemist J. Berzelius in 1823 gave a description of the silicon obtained by him by treating the potassium salt K 2 SiF 6 with metallic potassium at high temperature, however, only in 1854 silicon was obtained in crystalline form by A. Deville. Silicon is the second most abundant (after oxygen) element in the earth's crust, where it makes up more than 25% (mass). It occurs in nature mainly in the form of sand, or silica, which is silicon dioxide, and in the form of silicates (feldspars M (M = Na, K, Ba), kaolinite Al 4 (OH) 8, micas). Silicon can be obtained by calcining crushed sand with aluminum or magnesium; in the latter case, it is separated from the resulting MgO by dissolving magnesium oxide in hydrochloric acid. Technical silicon is obtained in large quantities in electric furnaces by reduction of silica with coal or coke. Semiconductor silicon is obtained by reduction of SiCl 4 or SiHCl 3 with hydrogen, followed by decomposition of the resulting SiH 4 at 400–600 ° C. High-purity silicon is obtained by growing a single crystal from a melt of semiconductor silicon according to the Czochralski method or by crucibleless zone melting of silicon rods. Elemental silicon is obtained mainly for semiconductor technology, in other cases it is used as an alloying additive in the production of steels and non-ferrous metal alloys (for example, to obtain ferrosilicon FeSi, which is formed by calcining a mixture of sand, coke and iron oxide in an electric furnace and is used as a deoxidizer and an alloying addition in the production of steels and as a reducing agent in the production of ferroalloys).
Application.
Silicon finds the greatest use in the production of alloys for giving strength to aluminum, copper and magnesium and for the production of ferrosilicides, which are important in the production of steels and semiconductor technology. Silicon crystals are used in solar cells and semiconductor devices - transistors and diodes. Silicon also serves as a raw material for the production of organosilicon compounds, or siloxanes, obtained in the form of oils, lubricants, plastics and synthetic rubbers. inorganic compounds silicon is used in the technology of ceramics and glass, as an insulating material and piezocrystals.
|
PROPERTIES OF SILICON |
|
| atomic number | 14 |
| Atomic mass | 28,086 |
| isotopes | |
| stable | 28, 29, 30 |
| unstable | 25, 26, 27, 31, 32, 33 |
| Melting point, °С | 1410 |
| Boiling point, °С | 2355 |
| Density, g / cm 3 | 2,33 |
| Hardness (Mohs) | 7,0 |
| Content in the earth's crust, % (wt.) | 27,72 |
| Oxidation states | –4, +2, +4 |
Properties.
Silicon is a dark gray, shiny crystalline substance, brittle and very hard, which crystallizes in the diamond lattice. It is a typical semiconductor (conducts electricity better than a rubber-type insulator, and worse than a conductor - copper). At high temperatures, silicon is highly reactive and reacts with most elements to form silicides, such as magnesium silicide Mg 2 Si, and other compounds, such as SiO 2 (silicon dioxide), SiF 4 (silicon tetrafluoride) and SiC (silicon carbide, carborundum). Silicon dissolves in a hot alkali solution with evolution of hydrogen: Si + NaOH ® Na 4 SiO 4 + 2H 2 . 4 (silicon tetrachloride) is obtained from SiO 2 and CCl 4 at high temperature; it is a colorless liquid, boiling at 58 ° C, easily hydrolyzed, forming hydrochloric (hydrochloric) acid HCl and orthosilicic acid H 4 SiO 4 (this property is used to create smoke inscriptions: HCl released in the presence of ammonia forms a white cloud of ammonium chloride NH 4 Cl) . Silicon tetrafluoride SiF 4 is formed by the action of hydrofluoric (hydrofluoric) acid on glass:
Na 2 SiO 3 + 6HF ® 2NaF + SiF 4 + 3H 2 O
SiF 4 is hydrolyzed, forming orthosilicic and hexafluorosilicic (H 2 SiF 6) acids. H 2 SiF 6 is close in strength to sulfuric acid. Many metal fluorosilicates are soluble in water (salts of sodium, barium, potassium, rubidium, cesium are slightly soluble), so HF is used to transfer minerals into solution when performing analyzes. The acid H 2 SiF 6 itself and its salts are poisonous.
Silicic acids.
Two silicon oxo acids H 4 SiO 4 (orthosilicon) and H 2 SiO 3 (metasilicic or silicic) exist only in solution and irreversibly turn into SiO 2 if water is evaporated. Other silicic acids are obtained due to different amounts of water in their composition: H 6 Si 2 O 7 (pyrosilicic acid from two molecules of orthosilicic acid), H 2 Si 2 O 5 and H 4 Si 3 O 8 (di- and trisilicic acids from two and, respectively, three molecules of metasilicic acid). All silicon acids are weak. When sulfuric acid silicate is added to a solution, a gel (gelatinous substance) is formed, which, when heated and dried, leaves a porous solid product - silica gel, which has a developed surface and is used as a gas adsorbent, desiccant, catalyst and catalyst carrier.
After oxygen silicon is the most abundant element in the earth's crust. It has 2 stable isotopes: 28 Si, 29 Si, 30 Si. Silicon is not found in free form in nature.
The most common are: salts of silicic acids and silicon oxide (silica, sand, quartz). They are part of mineral salts, mica, talc, asbestos.
Allotropy of silicon.
At silicon There are 2 allotropic modifications:
Crystalline (light gray crystals. The structure is similar to the crystal lattice of diamond, where the silicon atom is covalently bonded to 4 identical atoms, and is itself in sp3 - hybridization);
Amorphous (brown powder, more active form than crystalline).
silicon properties.
At temperature, silicon reacts with atmospheric oxygen:
Si + O 2 = SiO 2 .
If there is not enough oxygen (lack), then the following reaction may take place:
2 Si + O 2 = 2 SiO,
Where SiO- monoxide, which can also be formed during the reaction:
Si + SiO 2 = 2 SiO.
IN normal conditions silicon may react with F 2 , when heated - with Cl 2 . If the temperature is increased further, then Si will be able to interact with N And S:
4Si + S 8 = 4SiS 2;
Si + 2F 2 \u003d SiF 4.
Silicon is able to react with carbon, giving carborundum:
Si + C = SiC.
Silicon is soluble in a mixture of concentrated nitric and hydrofluoric acids:
3Si + 4HNO 3 + 12HF = 3SiF 4 + 4NO + 8H 2 O.
Silicon dissolves in aqueous solutions of alkalis:
Si + 2NaOH + H 2 O \u003d Na 2 SiO 3 + H 2.
When heated with oxides, silicon disproportionates:
2 MgO + 3 Si = mg 2 Si + 2 SiO.
When interacting with metals, silicon acts as an oxidizing agent:
2 mg + Si = mg 2 Si.
Application of silicon.
Silicon finds the greatest use in the production of alloys for giving strength to aluminum, copper and magnesium and for the production of ferrosilicides, which are important in the production of steels and semiconductor technology. Silicon crystals are used in solar cells and semiconductor devices - transistors and diodes.
Silicon also serves as a raw material for the production of organosilicon compounds, or siloxanes, obtained in the form of oils, lubricants, plastics and synthetic rubbers. Inorganic silicon compounds are used in ceramic and glass technology as an insulating material and piezocrystals.
Silicon (Si) is a non-metal that ranks second after oxygen in terms of reserves and location on Earth (25.8% in Earth's crust). In its pure form, it practically does not occur, it is mainly present on the planet in the form of compounds.
Silicon characteristic
Physical properties

Silicon is a brittle light gray material with a metallic tint or brown powdery material. The structure of a silicon crystal is similar to diamond, but due to differences in the bond length between atoms, the hardness of diamond is much higher.
Silicon is a non-metal available for electromagnetic radiation. Due to some qualities, it is in the middle between non-metals and metals:
With an increase in temperature to 800 ° C, it becomes flexible and plastic;
When heated to 1417 ° C, it melts;
Begins to boil at temperatures above 2600 ° C;
Changes density at high pressure;
It has the property of being magnetized against the direction of the external magnetic field(diamagnet).
Silicon is a semiconductor, and the impurities included in its alloys determine electrical characteristics future connections.
Chemical properties

When heated, Si reacts with oxygen, bromine, iodine, nitrogen, chlorine, and various metals. When combined with carbon, hard alloys with thermal and chemical resistance are obtained.
Silicon does not interact with hydrogen in any way, so all possible mixtures with it are obtained in a different way.
Under normal conditions, it reacts weakly with all substances except fluorine gas. Silicon tetrafluoride SiF4 is formed with it. Such inactivity is explained by the fact that a film of silicon dioxide forms on the surface of the non-metal due to the reaction with oxygen, water, its vapors and air and envelops it. Therefore, the chemical effect is slow and insignificant.
To remove this layer, use a mixture of hydrofluoric and nitric acids or aqueous solutions alkalis. Some special fluids for this include the addition of chromic anhydride and other substances.
Finding silicon in nature

Silicon is as important to the Earth as carbon is to plants and animals. Its crust is almost half oxygen, and if you add silicon to this, you get 80% of the mass. This connection is very important for the movement of chemical elements.
75% of the lithosphere contains various salts of silicic acids and minerals (sand, quartzites, flint, micas, feldspars, etc.). During the formation of magma and various igneous rocks, Si accumulates in granites and in ultramafic rocks (plutonic and volcanic).
There is 1 g of silicon in the human body. Most are found in bones, tendons, skin and hair, lymph nodes, aorta and trachea. It is involved in the process of growth of connective and bone tissues, and also maintains the elasticity of blood vessels.
The daily intake for an adult is 5-20 mg. Excess causes silicosis.
The use of silicon in industry

Since the Stone Age, this non-metal has been known to man and is still widely used.
Application:
It is a good reducing agent, so it is used in metallurgy to obtain metals.
Under certain conditions, silicon is able to conduct electricity, so it is used in electronics.
Silicon oxide is used in the manufacture of glasses and silicate materials.
Special alloys are used for the production of semiconductor devices.