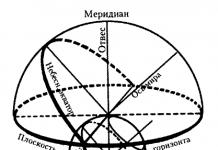
General reaction scheme:
The nucleophile gives up its pair of electrons to the substrate, due to which a new bond is formed, and the halogen leaves with its pair of electrons in the form of a halide anion. This happens alkylation nucleophile.
For nucleophilic substitution at a carbon atom in the sp 3 hybridization state, two main mechanisms have been established: bimolecular nucleophilic substitution (S N 2 ) And monomolecule nucleophilic substitution (S N 1 ).
Bimolecular nucleophilic substitution.
Bimolecular nucleophilic substitution is a synchronous process that occurs in one step. The breaking of the old connection and the formation of a new connection occur simultaneously. The nucleophile attacks the substrate from the side opposite to the leaving group (from the rear), and gradually displaces it from the molecule:
Y : + R-Hal ® ® Y-R + Hal -
transitional
state
S N 2 reactions have the following main features.
- Kinetic sign
The rate of the reaction depends on the concentration of both the substrate and the nucleophile. The reaction has a second overall order (first in the substrate and first in the nucleophile) and is described by the kinetic equation:
- v=k[Y]
- Stereochemical feature
If nucleophilic substitution occurs at an asymmetric carbon atom, then the configuration is reversed, since in the transition state the three non-reacting groups and the central carbon atom are in the same plane, and the incoming and outgoing groups are located on the same straight line, perpendicular to this plane. As a result, the structure turns inside out like an umbrella:
Monomolecule nucleophilic substitution.
Monomolecular nucleophilic substitution occurs in two stages:
At the first stage, under the influence of a solvent, heterolytic cleavage of the bond in the substrate occurs, resulting in the formation of a carbocation. The process proceeds slowly and determines the rate of the reaction as a whole. In the second step, the carbocation reacts rapidly with the nucleophile, yielding a substitution product.
The energy diagram of the process looks like:
S N 1 reactions have the following main features.
- Kinetic sign
The reaction rate depends only on the substrate concentration, since the nucleophile does not participate in the rate-limiting stage of the process. The reaction is first order and is described by the kinetic equation:
v=k
- Stereochemical feature
If nucleophilic substitution occurs at an asymmetric carbon atom, then, as a rule, a racemic mixture is formed, since attack by a nucleophile on a planar carbocation from both sides is equally probable:
Factors influencing the course of nucleophilic substitution
The ease of the reaction and its mechanism depend on many factors, among which are the following:
- structure of the hydrocarbon radical of the substrate;
- the nature of the leaving group;
- nucleophile strength;
- nature of the solvent.
Influence of the structure of the hydrocarbon radical.
The reactivity of primary, secondary and tertiary alkyl halides in nucleophilic substitution reactions is different, and the order of reactivity depends on the reaction mechanism.
The rate of reactions proceeding according to the S N 1 mechanism depends on the stability of the carbocation formed in the first stage of the reaction. Thus, the reactivity of alkyl halides in S N 1 reactions increases in the series:
which corresponds to the stability series of carbocations:
The success of the S N 2 reaction is determined by the efficiency of the nucleophile attack on the positively charged reaction center of the substrate. Therefore, electron-donating radicals R, by reducing the positive charge on the reaction center, slow down the nucleophilic attack. At the same time, an increase in the volume R makes it difficult for a nucleophile to approach the reaction center. The combined action of inductive and volumetric effects determines a number of reactivity of substrates in nucleophilic substitution reactions:
Allyl and benzyl halides have high reactivity, regardless of the reaction mechanism. In the S N 1 process they produce carbocations stabilized by conjugation:
Benzyl cation
The ease with which allyl and benzyl halides enter into S N 2-reactions are explained by the participation of multiple bonds in the stabilization of the transition state.
Influence of the nature of the leaving group.
The reactivity of alkyl halonides depends on the strength of the carbon-halogen bond, which decreases in the series:
C-F > C-Cl > C-Br > C-I.
It is equally important that the leaving group is thermodynamically stable. (It must be more stable than the nucleophile attacking the substrate). Good (relatively stable) leaving groups are weak bases. Halide anions are good leaving groups. Their relative stability increases as their basicity decreases in the series:
F-< Cl - < Br - < I -
In parallel, the reactivity of alkyl halides increases, regardless of which of the two mechanisms the reaction occurs:
RF< RCl < RBr < RI
Influence of the nature of the nucleophile.
Zerophilicity - is the ability of a particle to interact with a carbon atom bearing all or part of a positive charge. Nucleophilicity is a kinetic characteristic and is determined by the rate constants of the corresponding reactions.
Nucleophiles, like bases, can be strong or weak. There is no single scale of nucleophilicity, since the relative strength of the nucleophile can vary depending on the nature of the substrate and solvent. However, the following basic patterns can be identified.
1) Negatively charged nucleophiles are stronger than neutral molecules (their conjugate acids):
OH -> H 2 O; RO -> ROH; NH 2 -> NH 3
2) For elements of the same period, with increasing electronegativity of the atom, nucleophilicity decreases:
NH 2 -> OH -> F -
R 3 C - > RNH 2 - > RO - > F -
3) Electron-donating substituents increase, electron-withdrawing substituents decrease nucleophilicity. For example, the following reactivity series has been established for oxygen-containing nucleophiles:
RO - > OH - > ArO - > RCOO -
In the examples considered, the order of nucleophilicity of the reagents coincides with the order of their basicity and is explained by the same reasons. However, the strength of a nucleophile is determined not only by its basicity, but also polarizability.
4) For elements of the same subgroup, with increasing nuclear charge, nucleophilicity increases, despite a decrease in basicity:
RS --
I - - - -
An increase in nucleophilicity is associated with an increase in the polarizability of atoms and ions as their radius increases. The higher the polarizability of a nucleophile, the more easily its electron cloud is deformed and the more it is able to transfer electron density to the substrate.
This order of nucleophilicity can also be explained from the standpoint of the LMCO principle. Bronsted basicity is manifested in interaction with a hard acid H +, while nucleophilicity is manifested in interaction with a softer acidic center - a carbon atom, for which interaction with soft Lewis bases - RS - and I - will be preferable.
In addition, the relative strength of nucleophiles depends on the nature of the solvent. The smaller the size of the anion, the better it is solvated by polar protic solvents (i.e., solvents capable of forming hydrogen bonds with the anion), which reduces its reactivity. When changing the solvent, the order of reactivity of nucleophiles can be reversed.
In accordance with the S N 2 and S N 1 mechanisms, the nature of the nucleophile influences the course of the S N 2 reaction, since the nucleophile participates in the limiting (and only) stage of the process, and does not affect the rate of reactions proceeding according to the S N 1 mechanism, the limiting stage of which occurs without the participation of a nucleophile.
Influence of the nature of the solvent
The solvent affects the rate and mechanism of nucleophilic substitution reactions.
The reaction proceeds according to the S N 1 mechanism due to highly ionizing solvents. These include polar protic solvents (water, alcohols, carboxylic acids), since they solvate ionic intermediates well: a negatively charged leaving group - due to hydrogen bonds, a carbocation - due to free electron pairs.
The influence of the solvent on the S N 2 reaction is manifested to a lesser extent and depends on the charge distribution in the initial and transition states. As a rule, their rate decreases with increasing polarity of the solvent and increases when moving from protic to aprotic solvents (dimethylformamide, dimethyl sulfoxide, acetonitrile). In aprotic solvents, which are incapable of forming hydrogen bonds, the nucleophile (usually the anion) is less solvated and therefore stronger, which is important for the S N 2 reaction.
Thus, reactions proceed according to the mechanism S N 2 contribute to:
- substrate with a small volume hydrocarbon radical (primary);
- aprotic solvent;
- strong nucleophile.
Mechanism implementationsS N 1 contribute to:
- substrate with a branched hydrocarbon radical (tertiary);
- polar protic solvent;
- weak nucleophile.
Based on the ease of halogen substitution, regardless of the reaction mechanism, halogen derivatives are arranged in the following row:
allyl and benzyl halides > alkyl halides > vinyl and aryl halides
Halogen derivatives containing a bond (vinyl and aryl halides) have very low reactivity. The reaction proceeds by a different mechanism. The low mobility of halogen in vinyl and aryl halides is explained by an increase in the strength of the C-Hal bond due to the conjugation of a pair of halogen electrons with p-bond electrons:
Examples of nucleophilic substitution reactions
Nucleophilic halogen substitution reactions are widely used in organic synthesis. With their help you can replace halogen with others functional groups or hydrocarbon radicals and obtain any classes of organic compounds from halogen derivatives.
Examples of the synthetic use of halogenated aliphatic hydrocarbons are given in the table.
Table 7. S N -reactions of halogen derivatives
| Substrate | + | nucleophile | ® | product + leaving group |
| Preparation of alcohols | ||||
| R-Hal | + | OH - (H2O) | ® | R-OH + Hal - (HHal) |
| CH3Br | + | NaOH | CH3OH + NaBr | |
| (CH3)3CCl | + | H2O | ® | (CH 3) 3 COH + HCl |
| CH 2 =CH-CH 2 Cl | + | H2O | ® | CH 2 =CHCH 2 OH+HCl |
| Receipt ethers | ||||
| R-Hal | + | R/O- | ® | R-OR / + Hal - |
| CH 3 I | + | CH 3 CH 2 O - Na + | ® | CH 3 OCH 2 CH 3 + NaI |
| Receipt esters | ||||
| R-Hal | + | R/COO- | ® | R/COOR+Hal - |
| CH 3 CH 2 I | + | CH3COO-Na+ | ® | CH 3 COOCH 2 CH 3 + NaI |
| Preparation of thiols | ||||
| R-Hal | + | SH- | ® | R-SH + Hal - |
| CH 3 CH 2 Br | + | NaHS | ® | CH 3 CH 2 SH + NaBr |
| Preparation of sulfides | ||||
| R-Hal | + | R/S- | ® | R-SR/+Hal - |
| CH 3 CH 2 Br | CH 3 CH 2 S - Na + | ® | (CH 3 CH 2) 2 S + NaBr | |
| Preparation of amines and ammonium salts | ||||
| R-Hal | + | NH 2 - | ® | RNH 2 + Hal - |
| R-Hal | + | R/3N | ® | R R / 3 N + Hal - |
| Preparation of nitriles | ||||
| R-Hal | + | Сє N - | ® | R- Сє N + Hal - (S N 2) |
| CH 3 CH 2 Br | + | NaCN | ® | CH 3 CH 2 CN + NaBr |
| Preparation of nitro compounds | ||||
| R-Hal | NO 2 - | ® | R-NO 2 + Hal - (S N 2) | |
| CH 3 CH 2 I | AgNO2 | ® | CH 3 CH 2 NO 2 + AgI | |
| Preparation of halogen derivatives | ||||
| R-Hal | + | I - | ® | R-I + Hal - (S N 2) |
| CH3Cl | + | NaI | ® | CH 3 I + NaCl |
Vinyl and aryl halides are inert towards nucleophilic reagents. Halogen substitution in halobenzenes is only possible under very stringent conditions, for example:
Introduction of electron-withdrawing substituents into ortho- And pair-positions to the halogen activate halogenarenes in S N reactions:
Similarly, 2,4-dinitrofluorobenzene interacts with the amino groups of amino acids and peptides, which is used to determine their amino acid composition:
Nomenclature:
1) replacement (systematic),
2) radical-functional.
For the lower and most common representatives, trivial names are also acceptable, for example, fluoroform, chloroform, bromoform, iodoform, fluorotane.
Structure and properties

Depending on the nature of the halogen from fluorine to iodine, the polarity of the bond C–Hal decreases (since the electronegativity of the halogen decreases), but its polarizability increases and its length increases (since the radius of the halogen atom increases), and the bond strength decreases.
Since the polarizability of the bond C–I is greatest, then when iodoalkanes are dissolved in polar solvents, this bond is easily polarized up to heterolytic cleavage, that is, to heterolytic dissociation of the iodoalkane: R–I® R + + I- . Wherein Chemical properties compounds strongly depend on their polarizability.


Nucleophilic substitution reactions
Nucleophilic particles:
HO - ,RO - , - NH2, F - , Cl - , Br - ,I - ,CN - , H - , - CH2-R
H 2 O, ROH, NH 3, RNH 2, RR¢ NH, H2S, RSH
Mechanism of bimolecular nucleophilic substitution

Mechanism of monomolecular substitution
where II is a close ion pair
III - loose ion pair
IV and V - dissociated ions
Factors influencing the mechanism and rate of nucleophilic substitution
1. Influence of substrate structure.
bromomethane bromoethane 2-bromopropane
Speed S N 2-reactions:
 ,
,
Therefore, a high rate of nucleophilic substitution reactions can be characteristic of both primary and tertiary alkyl halides.
In the first case - due to the ease of interaction via S N 2-mechanism (free access to the reaction center, no steric hindrance),
in the second - by S N 1-mechanism (ease of substrate dissociation, stability of the resulting carbocation).
Secondary alkyl halides in most cases must react by a mixed mechanism, and their reaction rate will be relatively low, since there are obstacles to both monomolecular and bimolecular substitution.
2. Influence of the nature of the nucleophile.
3. Effect of solvents and catalysts.
4. Influence of the nature of the leaving group.
Examples of nucleophilic substitution reactions
1) Hydrolysis of haloalkanes- this is their transformation into alcohols according to the scheme:
R-X + H 2 O® R-OH + HX
Reaction mechanism: S N 1or S N 2 - determined mainly by the structure of the substrate, as well as other factors. For example, alkaline hydrolysis of bromoethane ( S N 2-mechanism):
Acid hydrolysis of 2-bromo-2-methylpropane ( S N 1-mechanism):

2) Alcoholysis of haloalkanes- this is the interaction of haloalkanes with metal alcoholates ( Williamson reaction), leading to the formation of ethers:
R-Hal + R¢ -O - Na+®R-O-R¢ + NaHal
Nucleophilic particle - alcoholate anion R¢ -O - .
Moreover, during the synthesis of mixed esters (with different R And R¢) it is necessary to make the correct choice of haloalkane and alcoholate ( RHal And R¢ -O- or R¢ Hal And R-O - - depending on the structure of hydrocarbon radicals) so that the reaction proceeds at the highest speed, and the possibility of alkene formation (the occurrence of a competing elimination reaction) is minimized.
3) Ammonolysis of haloalkanes- this is the interaction of haloalkanes with ammonia, leading to the production of amines (or their salts) - alkylation of amines according to Hoffmann
R-X + NH 3®[ R-NNH 3]+ X - R-NH 2 + NH 4 X
4) Replacing one halogen atom with another:
R-Br+I - ® R-I + Br -
An acidic environment and protic solvents promote the replacement of the fluorine atom,
and highly polar aprotic solvents, on the contrary, contain the iodine atom, since the nucleophilicity of halide ions decreases in the series I - >Br - >Cl - >F -
5) Interaction with cyanide is the interaction of haloalkanes with hydrocyanic acid salts, leading to the formation of organic cyanides (nitriles) or isocyanides. Cyanide ion is ambident a nucleophile, that is, it is capable of exhibiting its nucleophilic properties, both due to the carbon atom and due to the nitrogen atom:
- : Cº N® : C=N: -
Mechanism S N 1 - leads to the formation of isocyanides (isonitriles):
R + +: C=N: - ® R-N=C:
Mechanism S N 2:
This produces cyanides (nitriles).
6) Interaction with nitrites.
The nitrite anion is also an ambident nucleophile.
Therefore, its interaction with haloalkanes can lead to either nitro compounds or nitrous acid esters.
General reaction scheme:
R - NaI + :N - → R - N + :HaI -
The nucleophile gives up its pair of electrons to the substrate, due to which a new bond is formed, and the halogen leaves with its pair of electrons in the form of a halide anion. This happens alkylation nucleophile.
For nucleophilic substitution at a carbon atom in the sp 3 hybridization state, two main mechanisms have been established: bimolecular nucleophilic substitution (S N 2 ) And monomolecule nucleophilic substitution (S N 1 ).
Bimolecular nucleophilic substitution is a synchronous process that occurs in one step. The breaking of the old and the formation of a new connection occur simultaneously. The nucleophile attacks the substrate from the side opposite to the leaving group (from the rear), and gradually displaces it from the molecule:
N : + R-Hal → → N-R + Hal -
transitional
state
S N 2 reactions have the following main features:
- Kinetic
The rate of the reaction depends on the concentration of both the substrate and the nucleophile. The reaction has a second overall order (first in the substrate and first in the nucleophile) and is described by the kinetic equation:
2. Stereochemical
If nucleophilic substitution occurs at an asymmetric carbon atom, then the configuration is reversed, since in the transition state the three non-reacting groups and the central carbon atom are in the same plane, and the incoming and outgoing groups are located on the same straight line, perpendicular to this plane. As a result, the structure inverts like an umbrella.
Monomolecular nucleophilic substitution occurs in two stages.
At the first stage, under the influence of a solvent, heterolytic cleavage of the bond in the substrate occurs, resulting in the formation of a carbocation. The process proceeds slowly and determines the rate of the reaction as a whole. In the second step, the carbocation reacts rapidly with the nucleophile, yielding a substitution product.
S N 1 reactions have the following main features.
1. Kinetic
The reaction rate depends only on the substrate concentration, since the nucleophile does not participate in the rate-limiting stage of the process. The reaction is first order and is described by the kinetic equation:
2. Stereochemical feature
If nucleophilic substitution occurs at an asymmetric carbon atom, then, as a rule, a racemic mixture is formed, since attack by a nucleophile on a planar carbocation from both sides is equally probable.
Factors influencing the course of nucleophilic substitution
The ease of the reaction and its mechanism depend on many factors, among which are the following:
- structure of the hydrocarbon radical of the substrate;
- the nature of the leaving group;
- nucleophile strength;
- nature of the solvent.
Influence of the structure of the hydrocarbon radical.
The reactivity of primary, secondary and tertiary alkyl halides in nucleophilic substitution reactions is different, and the order of reactivity depends on the reaction mechanism.
The rate of reactions proceeding according to the S N 1 mechanism depends on the stability of the carbocation formed in the first stage of the reaction. Thus, the reactivity of alkyl halides in S N 1 reactions increases in the series: first<вторич< третич
which corresponds to the carbocation stability series.
The success of the S N 2 reaction is determined by the efficiency of the nucleophile attack on the positively charged reaction center of the substrate. Therefore, electron-donating radicals R, by reducing the positive charge on the reaction center, slow down the nucleophilic attack. An increase in the volume R makes it difficult for the nucleophile to approach the reaction center. The combined action of inductive and volumetric effects determines a number of reaction features of substrates in nucleophilic substitution reactions: primary > secondary > tertiary.
Allyl and benzyl halides have high reactivity, regardless of the reaction mechanism. In the S N 1 process, they form carbocations stabilized by p, π conjugation:
The ease with which allyl and benzyl halides enter into S N 2 reactions is explained by the participation of multiple bonds in stabilizing the transition state.
Influence of the nature of the leaving group.
The reactivity of alkyl halonides depends on the strength of the carbon-halogen bond, which decreases in the series:
C-F > C-Cl > C-Br > C-I.
It is equally important that the leaving group is thermodynamically stable. (It must be more stable than the nucleophile attacking the substrate). Good (relatively stable) leaving groups are weak bases. Halide anions are good leaving groups. Relative stability increases as their basicity decreases in the series:
F-< Cl - < Br - < I -
In parallel, the reactivity of alkyl halides increases:
RF< RCl < RBr < RI
Influence of the nature of the nucleophile.
Nullophilicity – is the ability of a particle to interact with a carbon atom bearing all or part of a positive charge. Nucleophilicity is a kinetic characteristic and is determined by the rate constants of the corresponding reactions.
Nucleophiles, like bases, can be strong or weak. There is no single scale of nucleophilicity, since the relative strength of the nucleophile can vary depending on the nature of the substrate and solvent. However, the following basic patterns can be identified:
1) negatively charged nucleophiles are stronger than neutral molecules (their conjugate acids):
OH -> H 2 O; RO -> ROH; NH 2 -> NH 3
2) for elements of the same period, with increasing electronegativity of the atom, nucleophilicity decreases:
NH 2 -> OH -> F -
R 3 C - > RNH 2 - > RO - > F -
3) electron-donating substituents increase, electron-withdrawing substituents decrease nucleophilicity. For example, the following reactivity series has been established for oxygen-containing nucleophiles:
RO - > OH - > ArO - > RCOO -
In the examples considered, the order of nucleophilicity of the reagents coincides with the order of their basicity and is explained by the same reasons. However, the strength of a nucleophile is determined not only by its basicity, but also polarizability.
4) For elements of the same subgroup, with increasing nuclear charge, nucleophilicity increases, despite a decrease in basicity:
I->Br->Cl->F-
An increase in nucleophilicity is associated with an increase in the polarizability of atoms and ions as their radius increases. The higher the polarizability of a nucleophile, the more easily its electron cloud is deformed and the more it is able to transfer electron density to the substrate.
This order of nucleophilicity can also be explained from the standpoint of the LMCO principle. Bronsted basicity is manifested in interaction with a hard acid H+, while nucleophilicity is manifested in interaction with a softer acidic center - a carbon atom, for which interaction with soft Lewis bases - RS- and I- will be preferable.
The relative strength of nucleophiles depends on the nature of the solvent. The smaller the size of the anion, the better it is solvated by polar protic solvents (i.e., solvents capable of forming hydrogen bonds with the anion), which reduces its reactivity. When replacing the solvent, the order of reactivity of nucleophiles can be reversed.
In accordance with the S N 2 and S N 1 mechanisms, the nature of the nucleophile influences the course of the S N 2 reaction, since the nucleophile participates in the limiting (and only) stage of the process, and does not affect the rate of reactions proceeding according to the S N 1 mechanism, the limiting stage of which occurs without the participation of a nucleophile.
Influence of the nature of the solvent
The solvent affects the rate and mechanism of nucleophilic substitution reactions.
The reaction proceeds according to the S N 1 mechanism due to highly ionizing solvents. These include polar protic solvents (water, alcohols, carboxylic acids), since they solvate ionic intermediates well: a negatively charged leaving group due to hydrogen bonds, a carbocation due to free electron pairs.
The effect of the solvent on SN2 reactions is less pronounced and depends on the charge distribution in the initial and transition states. As a rule, their rate decreases with increasing polarity of the solvent and increases when moving from protic to aprotic solvents (dimethylformamide, dimethyl sulfoxide, acetonitrile). In aprotic solvents, which are incapable of forming hydrogen bonds, the nucleophile (usually the anion) is less solvated and therefore stronger, which is important for the S N 2 reaction.
Thus, reactions proceed according to the mechanism S N 2 contribute to:
- substrate with a small volume hydrocarbon radical (primary);
- aprotic solvent;
- strong nucleophile.
Mechanism implementations S N 1 contribute to:
- substrate with a branched hydrocarbon radical (tertiary);
- polar protic solvent;
- weak nucleophile.
Based on the ease of halogen substitution, regardless of the reaction mechanism, halogen derivatives are arranged in the following row:
allyl and benzyl halides > alkyl halides > vinyl and aryl halides
Halogen derivatives containing a bond (vinyl and aryl halides) have very low reactivity. The reaction proceeds by a different mechanism. The low mobility of halogen in vinyl and aryl halides is explained by an increase in the strength of the C-Hal bond due to the conjugation of a pair of halogen electrons with electrons of π bonds:
Nucleophilic halogen substitution reactions are widely used in organic synthesis. With their help, it is possible to replace halogen with other functional groups or hydrocarbon radicals and obtain any classes of organic compounds from halogen derivatives.
Examples of the synthetic use of halogenated aliphatic hydrocarbons are given in the table.
Table. S N -reactions of halogen derivatives
| Substrate | + | nucleophile | → | product + leaving group |
| Preparation of alcohols | ||||
| R-Hal | + | OH - (H2O) | → | R-OH + Hal - (HHal) |
| CH3Br | + | NaOH | CH3OH + NaBr | |
| (CH3)3CCl | + | H2O | → | (CH 3) 3 COH + HCl |
| CH 2 =CH-CH 2 Cl | + | H2O | → | CH 2 =CHCH 2 OH+HCl |
| Preparation of ethers | ||||
| R-Hal | + | R/O- | → | R-OR / + Hal - |
| CH 3 I | + | CH 3 CH 2 O - Na + | → | CH 3 OCH 2 CH 3 + NaI |
| Preparation of esters | ||||
| R-Hal | + | R/COO- | → | R/COOR+Hal - |
| CH 3 CH 2 I | + | CH3COO-Na+ | → | CH 3 COOCH 2 CH 3 + NaI |
| Preparation of thiols | ||||
| R-Hal | + | SH- | → | R-SH + Hal - |
| CH 3 CH 2 Br | + | NaHS | → | CH 3 CH 2 SH + NaBr |
| Preparation of sulfides | ||||
| R-Hal | + | R/S- | → | R-SR/+Hal - |
| CH 3 CH 2 Br | CH 3 CH 2 S - Na + | → | (CH 3 CH 2) 2 S + NaBr | |
| Preparation of amines and ammonium salts | ||||
| R-Hal | + | NH 2 - | → | RNH 2 + Hal - |
| R-Hal | + | R/3N | → | R R / 3 N + Hal - |
| Preparation of nitriles | ||||
| R-Hal | + | С N - | → | R- С N + Hal - (S N 2) |
| CH 3 CH 2 Br | + | NaCN | → | CH 3 CH 2 CN + NaBr |
| Preparation of nitro compounds | ||||
| R-Hal | NO 2 - | → | R-NO 2 + Hal - (S N 2) | |
| CH 3 CH 2 I | AgNO2 | → | CH 3 CH 2 NO 2 + AgI | |
| Preparation of halogen derivatives | ||||
| R-Hal | + | I - | → | R-I + Hal - (S N 2) |
| CH3Cl | + | NaI | → | CH 3 I + NaCl |
Vinyl and aryl halides are inert towards nucleophilic reagents. Halogen substitution in halobenzenes is only possible under very severe conditions.
Introduction of electron-withdrawing substituents into ortho- And pair-positions to the halogen activate halogenarenes in S N reactions.
Elimination reactions
During the process of elimination (dehydrohalogenation), HHal is eliminated from a hydrogen halide molecule and an alkene is formed.
Cleavage occurs under the influence of strong bases - concentrated solutions of alkali metal hydroxides in alcohol, alcoholates or amides of alkali metals. The bases abstract a proton in the ß-position, and at the same time a halogen leaves the molecule in the form of a halide anion.
If the formation of two different elimination products is possible, then the alkene most substituted at the double bond is preferentially formed, which is thermodynamically more stable ( Zaitsev's rule):
Elimination reactions can occur by monomolecular (E1) or bimolecular (E2) mechanisms.
E1 reactions occur in parallel with S N 1 reactions and include two stages. First, a carbocation is formed, from which a proton is then removed by the action of a base.
E2 reactions occur in parallel with S N 2 reactions and include one stage, during which the simultaneous breaking of old bonds and the formation of new bonds occur.
The elimination mechanism (E1 or E2) is determined by the same factors as the corresponding nucleophilic substitution processes (SN1 and SN2).
Competition between nucleophilic substitution and elimination reactions
The processes of elimination and nucleophilic substitution always occur in parallel, since all nucleophiles are also bases.
The ratio of elimination and substitution products depends on the nature of the reagents and the reaction conditions. By selecting the reaction conditions and reagent, it is possible to achieve preferential reaction in the desired direction.
Factors contributing to the occurrence of cleavage:
1) High basicity of the reagent.
Strong bases will attack the hydrogen atom in the ß-position first rather than the carbon. Thus, under the influence of alcoholate anions, which are strong bases, elimination mainly occurs, while less basic thiolate anions react at the carbon atom and give substitution products:
CH 3 -CHI-CH 3 + C 2 H 5 O - Na + → CH 3 -CH=CH 2 + C 2 H 5 OH + NaI
CH 3 -CHI-CH 3 + C 2 H 5 S - Na + → (CH 3) 2 CH-S-C 2 H 5 +NaI
The process of elimination is facilitated not only by high basicity, but also by a large volume of the reagent, which makes it difficult to attack the carbon atom. Therefore, tertiary alcoholate anions provide mainly elimination products.
2) Low-polar solvents.
The same reagent, potassium hydroxide, reacts in an aqueous solution as a nucleophile to form substitution products, but in a less polar solvent, alcohol, it produces mainly elimination products.
3) High temperature.
Elimination reactions have a higher activation energy than substitution reactions, and therefore their rate increases more with increasing temperature.
4) The process of elimination is facilitated by a large volume of the hydrocarbon radical of the substrate, which makes it difficult for the reagent to attack the carbon atom. The tendency of halogen derivatives to elimination reactions increases in the following order:
primary< вторичные < третичные
The interaction of bases with tertiary alkyl halides leads mainly to elimination.
Lecture No. 11
ALDEHYDES AND KETONES
Aldehydes and ketones contain a carbonyl group, C=O. General formula:
Receipt methods.


Chemical properties.
Aldehydes and ketones are one of the most reactive classes of organic compounds. Their chemical properties are determined by the presence of a carbonyl group. Due to the large difference in the electronegativities of carbon and oxygen and the high polarizability of the π bond, the C=O bond has significant polarity (µ C=O = 2.5-2.8 D). The carbon atom of the carbonyl group carries an effective positive charge and is subject to attack by nucleophiles. The main type of reactions of aldehydes and ketones is nucleophilic addition reactions A N. In addition, the carbonyl group affects the reactivity of the C-H bond at the α-position, increasing its acidity.
Thus, the molecules of aldehydes and ketones contain two main reaction centers– C=O bond and C-H bond in the α position:

2.1. Nucleophilic addition reactions.
Aldehydes and ketones easily add nucleophilic reagents to the C=O bond. The process begins with an attack by a nucleophile on the carbonyl carbon atom. Then the tetrahedral intermediate formed in the first stage adds a proton and gives the addition product:

The activity of carbonyl compounds in A N reactions depends on the magnitude of the effective positive charge on the carbonyl carbon atom and the volume of substituents on the carbonyl group. Electron-donating and bulky substituents hinder the reaction; electron-withdrawing substituents increase the reactivity of the carbonyl compound. Therefore, aldehydes are more active in A N reactions than ketones:
The activity of carbonyl compounds increases in the presence of acid catalysts, which increase the positive charge on the carbonyl carbon atom:

Aldehydes and ketones add water, alcohols, thiols, hydrocyanic acid, sodium hydrosulfite, compounds like NH 2 X. All addition reactions occur quickly, under mild conditions, but the resulting products are, as a rule, thermodynamically unstable. Therefore, the reactions proceed reversibly, and the content of addition products in the equilibrium mixture can be low.
Connecting water.
Aldehydes and ketones add water to form hydrates. The reaction is reversible. The resulting hydrates are thermodynamically unstable. The equilibrium is shifted towards addition products only in the case of active carbonyl compounds.

The hydration product of trichloroacetic aldehyde, chloral hydrate, is a stable crystalline compound that is used in medicine as a sedative and hypnotic.
Addition of alcohols and thiols.
Aldehydes combine with alcohols to form hemiacetals. With an excess of alcohol and in the presence of an acid catalyst, the reaction proceeds further - until acetals

The reaction to form a hemiacetal occurs as a nucleophilic addition and is accelerated in the presence of acids or bases.

The process of acetal formation occurs as a nucleophilic substitution of the OH group in the hemiacetal and is possible only under conditions of acid catalysis, when the OH group is converted into a good leaving group (H 2 O).

The formation of acetals is a reversible process. IN acidic environment Hemiacetals and acetals are easily hydrolyzed. In an alkaline environment, hydrolysis does not occur. The formation and hydrolysis reactions of acetals play an important role in carbohydrate chemistry.
Ketones do not produce ketals under similar conditions.
Thiols, as stronger nucleophiles than alcohols, form addition products with both aldehydes and ketones.
Addition of hydrocyanic acid
Hydrocyanic acid combines with carbonyl compounds under basic catalysis to form cyanohydrins.

The reaction has preparative value and is used in the synthesis of α-hydroxy- and α-amino acids (see lecture No. 14). The fruits of some plants (for example, bitter almonds) contain cyanohydrins. Hydrocyanic acid released during their breakdown has a poisonous effect.
Addition of sodium bisulfite.
Aldehydes and methyl ketones add sodium bisulfite NaHSO 3 to form bisulfite derivatives.

Bisulfite derivatives of carbonyl compounds – crystalline substances, insoluble in excess sodium bisulfite solution. The reaction is used to isolate carbonyl compounds from mixtures. The carbonyl compound can be easily regenerated by treating the bisulfite derivative with an acid or alkali:
Interaction with compounds of the general formula NH 2 X.
Reactions proceed according to the general scheme as an addition-elimination process. The addition product formed at the first stage is not stable and easily splits off water:

According to the given scheme, ammonia, primary amines, hydrazine, substituted hydrazines, and hydroxylamine react with carbonyl compounds:

The resulting derivatives are crystalline substances that are used for the isolation and identification of carbonyl compounds.
2.2. Reactions at the α-carbon atom.
Keto-enol tautomerism.
Hydrogen in the α-position to the carbonyl group has acidic properties, since the anion formed upon its elimination is stabilized due to resonance:

The result of the proton mobility of the hydrogen atom in the α-position is the ability of carbonyl compounds to form enol forms due to the migration of a proton from the α-position to the oxygen atom of the carbonyl group:

Ketone and enol are tautomers. Tautomers are isomers that can quickly and reversibly transform into each other due to the migration of a group (in this case, a proton). The equilibrium between a ketone and an enol is called keto-enol tautomerism.
Most carbonyl compounds exist predominantly in the ketone form. The content of the enol form increases with increasing acidity of the carbonyl compound, as well as in the case of additional stabilization of the enol form due to hydrogen bond or through pairing.
Table 8. Content of enol forms and acidity of carbonyl compounds
For example, in 1,3-dicarbonyl compounds, the mobility of the protons of the methylene group increases sharply due to the electron-withdrawing effect of two carbonyl groups. In addition, the enol form is stabilized due to the presence of a system of conjugated α-bonds and an intramolecular hydrogen bond:

Enolization and the formation of enolate anions are the first stages of reactions of carbonyl compounds occurring at the α-carbon atom. The most important of them are halogenation And aldol-crotonic condensation.
Halogenation
Aldehydes and ketones easily react with halogens (Cl 2, Br 2, I 2) to form α-halogen derivatives:

The reaction is catalyzed by acids or bases. The reaction rate does not depend on the concentration and nature of the halogen. The process proceeds through the formation of the enol form (slow stage), which then reacts with a halogen (fast stage). Thus, the halogen is not involved in the rate-determining step of the process.

If a carbonyl compound contains several α-hydrogen atoms, then the replacement of each subsequent one occurs faster than the previous one due to an increase in their acidity under the influence of the electron-withdrawing effect of the halogen. In an alkaline environment, acetaldehyde and methyl ketones give trihalogen derivatives, which are then decomposed by excess alkali to form trihalomethanes ( haloform reaction):
Condensation reactions
In the presence of catalytic amounts of acids or alkalis, carbonyl compounds containing α-hydrogen atoms undergo condensation to form α-hydroxycarbonyl compounds.

In education S-S connections involve the carbonyl carbon atom of one molecule ( carbonyl component) and an α-carbon atom of another molecule ( methylene component). This reaction is called aldol condensation(by the name of the condensation product of acetaldehyde - aldol).
When the reaction mixture is heated, the product easily dehydrates to form an α,ß-unsaturated carbonyl compound:

This type of condensation is called croton(by the name of the condensation product of acetaldehyde - crotonaldehyde).
Not only carbonyl compounds, but also other C-H acids can act as the methylene component in condensation reactions. Condensation reactions have preparative value, as they allow the chain of carbon atoms to be increased. Many biochemical processes occur by the type of aldol condensation and retroaldol decomposition (reverse process): glycolysis, synthesis of citric acid in the Krebs cycle, synthesis of neuraminic acid.
Before considering each of the classes of acid derivatives separately, it is useful to give a general picture of their behavior, within which it will be easier to consider the rather numerous individual characteristics.
Each derivative is almost always obtained - directly or indirectly - from the corresponding carboxylic acid, and can be converted back to the carboxylic acid by simple hydrolysis. An important role in the chemistry of acid derivatives is played by their transformation into each other and into the original acid. In addition, each class has its own characteristic reactions.
Derivatives carboxylic acids, like the acids themselves, contain a carbonyl group. This group is retained in the products of most reactions of these compounds and does not undergo visible changes. However, the very presence of this group in the molecule determines the characteristic reactivity of these compounds, and this fact is key to understanding their chemistry.
Acyl compounds (carboxylic acids and their derivatives) typically undergo nucleophilic substitution reactions in which or groups are replaced by other basic groups. Substitution occurs much more easily than substitution at a saturated carbon atom; in fact, many of these reactions do not occur at all in the absence of a carbonyl group, such as substitution with

To explain the properties of acyl compounds, it is necessary to return to the structure of the carbonyl group. We have already met this group
when studying aldehydes and ketones (Sections 19.1 and 19.9) and we know what reactions can be generally expected for it.
The carbon of the carbonyl group is bonded to three other atoms by -bonds; Because these bonds use -orbitals (Section 2.23), they lie in a plane at an angle of 120° (2.094 rad) to each other. The remaining -orbital of the carbon atom overlaps with the -orbital of the oxygen atom to form an -bond; carbon and oxygen are thus connected by a double bond. The part of the molecule immediately adjacent to the carbon atom of the carbonyl group is flat; oxygen, carbon of the carbonyl group and two atoms associated with it lie in the same plane

Both electronic and steric factors make the carbonyl group particularly accessible to nucleophilic attack on the carbonyl group carbon. These factors are the following: a) the tendency of oxygen to gain electrons, even if this causes a negative charge on the oxygen; b) the relative ease of the transition state during the transformation of a trigonal reagent into a tetrahedral intermediate. The same factors make acyl compounds accessible to nucleophilic attack.
However, acyl compounds differ from aldehydes and ketones in the nature of the second stage of the reaction. A tetrahedral intermediate resulting from an aldehyde or ketone adds a proton to form the addition product. The tetrahedral intermediate formed from the acyl compound eliminates the group, resulting in a trigonal compound again, and the reaction results in substitution.

It is possible to understand why these two classes of compounds behave differently. The ease with which a group is eliminated depends on its basicity: the weaker the base, the easier the group is eliminated. For acid chlorides, acid anhydrides, esters and amides, the group is respectively the following: very weak foundation moderately weak base and strong bases and But in order for substitution to occur with aldehydes or ketones, the group being eliminated must be a hydride ion or alkyl ion which, as we know, are the strongest bases (note very low acidity and As a result in reactions with aldehydes and ketones, instead of elimination, addition always occurs.
(see scan)
(see scan)
Thus, nucleophilic substitution in the acyl group occurs in two stages with the intermediate formation of a tetrahedral intermediate. Usually the overall speed is determined by the speed of both stages, but the first stage is more important.
The rate of the first stage (formation of a tetrahedral intermediate) is determined by the same factors as the addition reaction to aldehydes and ketones (Section 19.9): it is favored by the effect of electron withdrawal, which stabilizes the resulting negative charge; it is hampered by the presence of voluminous groups that create spatial obstacles in the transition state. The ease of the second stage depends on the basicity of the leaving group
Homolytic (radical) reactions
For example, halogenation of alkanes (chain reaction)
CH 4 + Cl 2 hν → CH 3 Cl + HCl (stage 1);
CH 3 Cl + Cl 2 hν → CH 2 Cl 2 + HCl (stage 2);
CH 2 Cl 2 + Cl 2 hν → CHCl 3 + HCl (3rd stage);
CHCl 3 + Cl 2 hν → CCl 4 + HCl (4th stage).
Attention! In alkane substitution reactions, hydrogen atoms are most easily replaced at tertiary carbon atoms, then at secondary carbon atoms, and, lastly, at primary carbon atoms.
CH 3 - CH 2 - CH - CH 3 + Cl 2 hν → mixture of haloalkanes.
1; 4 – primary; 3 – secondary; 2 – tertiary.
Heterolytic (ionic)
Heterolytic decomposition of a covalent polar bond leads to the formation of nucleophiles (anions) and electrophiles (cations):
b) H 2 O → H + + OH -
The resulting ions undergo further transformations, for example:
CH 3 + + OH - → CH 3 OH
electrophile nucleophile
Ionic reactions are divided according to the nature of the reagent acting on the molecule into electrophilic and nucleophilic.
Electrophile E(electron loving) – this is a particle that attacks the carbon atom of an organic compound, taking away an electron pair from it (it is an electron acceptor). Examples of particles - electrophiles: H 3 O +, H +, HCl, HNO 3, NO 2 +, AlCl 3, etc.
Nucleophile N(nucleus loving) is a particle that attacks a carbon atom, providing it with an electron pair (is an electron donor). Such particles, as a rule, have basic properties. These include: OH -, Cl -, S 2-, NH 3, H 2 O, R-OH, CH 3 O - and others
Nucleophilic reactions- these are reactions organic matter with nucleophiles, i.e. anions or molecules that provide an electron pair to form a new bond:
CH 3 Br (substrate) + NaOH (nucleophile reagent) → CH 3 OH + NaBr
Electrophilic reactions– reactions of organic compounds with electrophilic reagents, i.e. cations or molecules that have an empty orbital, ready to accept an electron pair to form a new bond
C 6 H 6 (substrate) + HO: - NO 2 + (electrophile reagent) → C 6 H 5 – NO 2 + H –OH
Examples of nucleophilic reactions
Nucleophilic substitution:
Not for all reactions it is possible to clearly determine the mechanism by which they occur, since pure S N 1 or S N 2 are just ideal (limiting) model cases. It should be remembered that the same substrate can react with the same nucleophile, depending on the reaction conditions and solvent, as in the mechanism S N 1, so S N 2.
For example, the rate of hydrolysis of 2-bromopropane is described taking into account the mixed mechanism of its occurrence:
CH 3 −CHBr−CH 3 + HO − → CH 3 −CHOH−CH 3 + Br −
4.Alkanes-organic. compounds whose molecules consist of carbon and hydrogen are classified as hydrocarbons. If a hydrocarbon molecule contains only simple sigma bonds, and their composition corresponds to the general formula C n H 2 n +2, then they are classified as saturated, or paraffins. Carbon atoms in alkanah find. in a state of sp 3 hybridization and are tetravalent. Alkanes form a series of homologues, in which each subsequent member differs from the previous one by homology. The difference is the CH2 group.
Isomerism: 1) isomerism of the carbon skeleton;
2) isomerism of the position of the substituent in the carbon chain
Nomenclature: according to nomencl. IUPAC names of saturated hydrocarbons are characterized by the suffix –an-. The first four hydrocarbons have trivial names, and starting from the fifth, they are based on the name of the Latin numeral in accordance with the number of at. carbons in the molecule. The names of hydrocarbon radicals are constructed by replacing the suffix –an on –il.
General methods of obtaining:
1. Wurtz reaction (interaction of halogenated hydrocarbons with alkaline Me-Li, Na, K)
CH3Br+2Na +Br-CH32NaBr+CH3-CH3
CH3-Cl+2Na+Cl-CH3CH3-CH3+2NaCl
2.Hydrogenation of unprepared hydrocarbons
CH2=CH2-ethylene
CH2=CH2+H2 Pt,t CH3-CH3-ethane
Physical properties:
1.from C1-C4 gases (b.z)
2. from C5-C22 liquid (special)
3.>C22-solid substances (b.z)
Well studied up to C100. Their boiling or melting temperatures in the homologous series increase monotonically with each new –CH2 group (a striking example of the transition from quantity to quality).
Chemical properties:
1.R-tions of mixing at.N:
A) direct halogenation (F2.Cl2.Br2)
Cl2+CH4 hv HCL+CH3Cl+CH2Cl2+CHCl3+CCl4
Fluoridation (with explosion)
Chlorination (in the light)
Chlorination mechanism (chain, radical)
Cl2 hv 2Cl .
Cl . +CH4HCl+CH3
CH3+Cl . CH3Cl-chain break
CH3 . +Cl2Cl . +CH3ClHCl+CH2Cl . etc-chain growth
Bromination (heating, receiving light)
B) nitration (-No2 gr.)-tion Konovalava
CH3-CH3+NO3 140C H2O+CH3-CH2NO2 nitroethane
B) Sulfochlorination (SO3, Cl2)
CH3-CH3+SO3+CL2 hv HCl+CH3-CH2-SO3Cl sulfachloroethane
D) Creting
CH3-CH2-CH2-CH3 Pt,t CH2=CH2+CH3-CH3
Use in agriculture: distribution of waste oils as herbicides to destroy dicotyledonous weeds in cereals and corn crops. Large practical significance purchased oil waste due to the discovery of the possibility of using it as an organic material. Substrates for growing certain strains of yeast cultures for the production of dry protein-vitamin concentrates (PVC).
Sp3 hybridization
Occurs when one s- and three p-orbitals mix, forming four sp3-hybrid orbitals of equal shape and energy. They can form four σ bonds with other atoms or be filled with lone pairs of electrons.
The axes of sp3 hybrid orbitals are directed towards the vertices of a regular tetrahedron. The tetrahedral angle between them is 109°28", which corresponds to the lowest electron repulsion energy. Also, sp3 orbitals can form four σ bonds with other atoms or be filled with lone pairs of electrons. This state is typical for carbon atoms in saturated hydrocarbons and, accordingly, in alkyl radicals and their derivatives.
Examples of compounds characterized by sp 3 hybridization: NH 3, POCl 3, SO 2 F 2, SOBr 2, NH 4+, H 3 O +. Also, sp 3 hybridization is observed in all saturated hydrocarbons(alkanes, cycloalkanes) and other organic compounds: CH 4, C 5 H 12, C 6 H 14, C 8 H 18, etc. General formula of alkanes: C n H 2n+2. The general formula of cycloalkanes is C n H 2n. In saturated hydrocarbons everything chemical bonds single, therefore only σ-overlap is possible between the hybrid orbitals of these compounds.
sp 3 - Hybridization is characteristic of carbon atoms in saturated hydrocarbons (alkanes) - in particular in methane

Fig. 2 Diagram of the electronic structure of a methane molecule
6. Alkenes are organic compounds whose molecules consist of at. carbon and hydrogen and, in addition to simple sigma-st. content Also double pi-st. Their composition corresponds to the general formula CnH2n. which means the presence. In their composition. Molek. Deficiency 2 at. Hydrogen versus alkanes.
Ethylene CH2=CH2
Electronic nature of a double bond: From the point of view of electronic concepts, a double bond is carried out by two pairs of electrons belonging to the two carbon atoms being bonded. One pair of electrons forms an ordinary covalent σ bond, while the second pair of electrons forms a bond of a different nature, the so-called π bond. The special configuration of the electron clouds of the π bond determines the fixation of the directions of the remaining four covalent σ bonds at these two carbon atoms. These bonds turn out to lie in the same plane and are located at angles of 120° relative to each other and relative to the direction of the σ-bond between the carbon atoms connected by a double bond. A double bond is not twice as strong energetically as a single bond. The corresponding bond energies for C-C and C=C are 79.3 and 140.5 kcal/mol.
Isomerism:
1.carbon skeleton
CH2=CH-CH2-CH2-CH3 pentene-1
CH2=C(CH3)-CH2-CH3 2-methylbutene-1
2. Deputy position
CH2=CH-CH(CH3)-CH3 3-methylbutene-1
3. position of the double bond in the hydrocarbon chain
CH3-CH=CH-CH2-CH3 pentene-2
4. geometric (cis-, trans-)
Nomenclature:
They use IUPAC nomencl. A distinctive feature is the need to select, in the case of alkenes, the main carbon-carbon chain, which includes a double-stranded character. Alkanes suffixes –vn and alkenes are replaced with –en. For example:
Methods of obtaining:
1. Cracking of alkanes. Consists in the thermal decomposition of alkanes with a longer chain of carbon atoms to a mixture of alkanes and unsaturated hydrocarbons with a short chain and hydrogen at 500-700 C and high pressure:
2. Dehydration of alcohols. Occurs in the presence of a catalyst - aluminum oxide and water-removing agents with mandatory additional heating and in accordance with the scheme (according to A. Zaitsev’s rule: the elimination of water from alcohols occurs with the participation of the hydroxyl group due to the hydrogen at. hydrogenated at.carbon):
3. Dehalogenation of dihalogen derivatives of hydrocarbons occurs in the presence of active divalent metals (Mg, Zn) when heated, according to the scheme:
4. Reduction of alkynes (saturation of the triple bond with active hydrogen), depending on the type of catalyst used, leads to the formation of cis- or trans-alkenes according to the following scheme:
5. Dehydrohalogenation of monohalohydrocarbons with alcohol alkali occurs at temp. Boiling alcohol
Sp2 hybridization
Occurs when one s- and two p-orbitals mix. Three hybrid orbitals are formed with axes located in the same plane and directed to the vertices of the triangle at an angle of 120 degrees. The non-hybrid p-atomic orbital is perpendicular to the plane and, as a rule, is involved in the formation of π bonds
Carbon atoms in the sp 2 hybrid state form allotropic forms such as graphite, graphene, fullerenes and other nanostructures.
sp 2 -Hybridization is typical for atoms C, N, O, etc. with a double bond (sp 2 atoms are highlighted in red): H 2 C=C H 2 (animation, 21.3 Kb), H 2 C=C HR, R 2 C=N R,
R -N=N -
R, R 2 C=O, R -N=O, as well as for cations like R 3 C + and free radicals R 3 C.
Electronic model of the ethylene molecule.

Cis-, trans-isomerism using the example of butene -2.
Cis-butene2, trans-butene2
Geometric, or cis-trans, isomerism is a type of spatial isomerism that depends on the different arrangement of atoms relative to the plane of the double bond. A dis-isomer is an isomer in which identical atoms (or atomic groups) are located on the same side of the double bond plane.
8. Alkynes are organic compounds whose molecules are composed of. Their carbon and hydrogen atoms and, in addition to simple sigma bonds, also contain at least two double pi bonds; there is one triple bond. Their composition corresponds to the general formula CnH2n-2, which means that in comparison with alkanes there is a deficiency of 4 at once .hydrogen in a molecule.
Acetylene C2H2();propyne()
Nature of the triple bond:
In alkynes, the −C≡C− bond is linear (angle 180°) and located in the same plane. Carbon atoms are connected by one σ- and two π-bonds, the maximum electron density of which is located in two mutually perpendicular planes. The length of the triple bond is approximately 0.121 nm, the binding energy is 836 kJ/mol.


The diagram above shows molecular orbitals ethylene and acetylene.
Isomerism:
1.carbon skeleton
2. position of deputy
3. Position of the triple bond (this type of isomerism is impossible for this type of chain, since it does not require the length of the corner chain)
Nomenclature: IUPAC. In this case, the ending is in, characterizing the presence of a triple bond:
Methods of obtaining:
1.Dehydrogenation of alkenes
CH2=CH2 ethylene Kt,t H2+HC=CH austilene
2.Double dehydrogenation of dihalohydrocarbons (-2HX)
3.Carbide method (only for acitylene)
A)>60% in the chemical industry
B)>30% in technology>3000С
Chemical properties:
1. Reactions of substitution of H atoms at at. C with triple st.
A) substitution with metal (Na,k,Cu)
B) substitution with halogen (Cl, Br)
2. Reactions involving pi-st.
A) joining
Connection with water (Kucherova river)
3. Polymerization processes
A) dimerization
Question 9. Sp-hybridization. electronic model of acetylene molecule. qualitative reaction to acetylene. sp-hybridization (typical of alkynes). Occurs when one s- and one p-orbital are mixed. Two equivalent sp-atomic orbitals are formed, located linearly at an angle of 180 degrees and directed in different directions from the nucleus of the carbon atom. The two remaining non-hybrid p-orbitals are located in mutually perpendicular planes and participate in the formation of π-bonds, or occupy lone pairs of electrons. The simplest member and founder is homologous to the series u/v-acetylene C2H2. The C atoms in acetylene are united by three common pairs of electrons, i.e. connected by a triple C===C bond. The structure of acetylene is: n-s===s-n. Based on the SP-hybrid state of the AT at the triple bond, the structure of the acetylene molecule can be represented as the result of the overlap of 2 hybrid (s and p x) orbitals from each neighboring AT. In this case, the hybrid sp-orbitals are located on the same straight line, forming an angle of 180. (see the structure of mol acetylene in the uch and tet) the presence of two P-bonds and sp-hybrid orbitals leads to a sharp increase in the electron density between at H. (see fig. tet) as a consequence weak connection S-N.
Preparation methods: dehydrogenation of alkenes
Carbide method (for acetylene)
1. Substitution reaction of atH at at C triple light: a) substitution with Me(Cu, Hg)
(alkyne solution Cu-quality solution for these alkynes)
Substitution with halogen (chlorine, bromine)
Question 10. Chemistry of alkenes and alkynes.
Alkenes. 1. Additions: a) halogens
This is a qualitative reaction to a double bond
B) hydrogen halides
Markovnikov's rule: any electrophilic particle will attach to a molecule of an unsymmetrical alkene at the site where the n-bond is broken to a more hydrogenated at C,a process proceeds through the stage of formation of the most stable carbocation.
D) oxidative hydroxylation (Wagner solution)
This method is often used to detect double bonds (qualitative method)
2. polymerization-combination large number identical or different molecules into one new large molecule.
3.atH substitution
Alkynes 1. Substitution of at H at the triple bond: a) substitution with Me(K, Na, Cu)
B) substitution with halogen
R-iya taking into account P-connection a) connection r-iya
B) joining with NON (Kucherov district)
3rd stage of polymerization: a) dimerization I
B) dimerization II
Their significance: of the individual unsaturated hydrocarbons, the biogenic value of ethylene CH2 = CH2 should be noted. The processes of ripening of fruits and berries are accompanied by the obligatory formation of ethylene in their peel. The ability of ethylene to stimulate root formation processes and cause leaf fall in some plants has been noted. Ethylene leads to a noticeable acceleration of their ripening. The simplest alkyne, acetylene, has a similar property.
Question 11. Cycloalkanes are cyclic saturated hydrocarbons. СnH2n-general formula. (see their structure in tet)
Bayer's cycle stress theory suggests that the C atoms in cycloalkanes form a flat closed cycle, in which the bond angles of a simple C-C bond differ from the tetrahedral, least strained bond angle in the methane molecule. Moreover, the more the bond angle in cycloalkanes differs from the tetrahedral one, the more stressed their molecular cyclic skeletons are. According to Bayer, the voltage value should decrease from cyclopropane to cyclopentane, and then increase again in cyclohexane. Molecule conformation is the spatial arrangement of atoms in a molecule of a certain configuration, caused by rotation around one or more single sigma bonds. The rings in cycloalkanes (with the exception of cyclopropane) are non-planar. Thus, cyclobutane has a slightly swollen shape - one of the carbon atoms is located above or below the plane in which the other three atoms are located, cyclopentane has an envelope conformation or a twist conformation, cyclohexane can exist in two chair conformations, during the transition between which (via the bath conformation ) all axial substituents become equatorial and vice versa. For larger cycles, the number of conformations increases, so such compounds exist in the form of several interconverting conformers. Thus, for cycloheptane, 4 stable conformations are possible: distorted chair (twist chair), chair, bath, distorted bath (twist bath), for cyclooctane - 11 conformations.
Chem sv-va: (lecture and study)
Question 12 Arena- these are hydrocarbon derivatives of benzene, including benzene itself (C6H6). Benzene was first discovered by Faraday.
The simplest representatives (single-core arenas):

Multi-core arenas: naphthalene C 10 H 8, anthracene C 14 N 10 and etc.

The concept of aromaticity: term " aromatic compounds" arose a long time ago due to the fact that some representatives of this series of substances have a pleasant odor. However, nowadays the concept of "aromaticity" has a completely different meaning. The aromaticity of a molecule means its increased stability, due to the delocalization of π-electrons in the cyclic system. To aromatic The compounds include benzo and substances that resemble it in their chemical behavior.
Hückel's rule According to cat, any organic compound that satisfies the following conditions will be aromatic: 1. the presence of a closed and oplanar (flat) cycle. 2. continuity of conjugation of P-electrons of all P-bonds. 3. the number of P-electrons participating in the conjugation must correspond to the formula: 4n+2(n-integer).
Receipt methods: 1. Dehydrogenation of the corresponding cycloalkanes successfully occurs over a Pt catalyst at a temperature of approximately 300C.
2.aromatization of alkanes
3. trimerization of alkynes
The theory of electrophilic substitution: electrophilic substances with electron deficiency, as well as acids. Electrophilic substitution reactions are substitution reactions in which the attack is carried out by an electrophile particle that is positively charged or has a deficiency of electrons.
1.p-ii substitution at.H of the benzene ring.
A) halogenation
B) alkylation (Friedel-Crafts solution)
2nd connection on the benzene ring
A) hydrogenation.
B) chlorination
3. oxidation of alkyl derivatives of benzene.
Question 13. Alcadienes. Dienes are organic compounds, molecules composed of at. C and H, in addition to simple b-bonds, also contain two double P-links. Their general formula is СnH2n-2.
Classification dienes is based on the relative position of 2 double C=C bonds in their molecules. Based on this feature, they fall into the following groups: 1. cumulated – dienes with adjacent arrangement of two P-bonds, which have the general formula: R-CH=C=CH2. The most common representative is allene CH2=C=CH2, which is why they are also called allenes. 2. conjugated dienes with an alternating arrangement of bonds and the general formula: R-CH=CH-CH=CH2. 3. Isolated dienes with a distance between P-strings exceeding one simple b-string, with total. formula: R-CH=CH-(CH2)n-CH=CH2, where n=1.2 or more.
IUPAC nomencl.: the choice of the main chain of carbon atoms and the numbering of atoms is carried out so that the positions of double bonds are designated by the smallest numbers, and to indicate the number of double bonds, the suff.-diene is used. For example:

The simplest member of conjugated dienes is butadiene: CH2=CH-CH=CH2. Four at.C in butadiene are united by common pairs of electrons, forming two, alternating with a simple b-st, double P-st. This is common distinctive feature structures of the entire class of conjugated dienes. Hydrocarbons with conjugated double bonds are obtained: 1) by dehydrogenation of alkanes contained in natural gas and oil refining gases, when passing them over a heated catalyst
CH 3 –CH 2 –CH 2 –CH 3 –– ~600°С; Cr 2 O 3, Al 2 O 3- CH 2 =CH–CH=CH 2 + 2H 2
2)dehydrogenation and dehydration ethyl alcohol when passing alcohol vapor over heated catalysts (method of Academician S.V. Lebedev
2CH 3 CH 2 OH –– ~450° C; ZnO,Al2O3 CH 2 =CH–CH=CH 2 + 2H 2 O + H 2,
He was the first to produce butadiene rubber based on butadiene.
The interaction of two or more neighboring p-bonds to form a single p- electronic cloud, resulting in the transfer of mutual influence of atoms in this system, is called the conjugation effect.
Let us consider the reactions of halogenation and hydrohalogenation of conjugated dienes. 
Divinyl and isoprene undergo polymerization and copolymerization (i.e., co-polymerization) with other unsaturated compounds, forming rubbers. Rubbers are elastic high-molecular materials (elastomers), from which rubber is produced by vulcanization (heating with sulfur). Polymerization reactions. Diene hydrocarbons have an extremely important feature: they easily enter into polymerization reactions to form rubber-like high-molecular products. Polymerization reactions occur with the addition of molecules to each other in the 1,4- or 1,2-position, as well as with simultaneous addition in the 1,4- and 1,2-positions.
Butadiene is a gas (bp -4.5°C), isoprene is a liquid, boiling at 34°C, dimethylbutadiene is a liquid, boiling at 70°C. Isoprene and other diene hydrocarbons are capable of polymerizing into rubber. Natural rubber in its purified state is a polymer with general formula(C5H8)n and is obtained from the milky sap of some tropical plants
Question14.polymerization reactions of diene hydrocarbons.
The process of polymerization leads to the formation of polymers from monomer molecules as a result of the rupture of the main valences of weak P-bonds and the sequential binding of the resulting radicals to each other. Polymerization of diene hydrocarbons. The production of synthetic rubber is the main area of application of diene hydrocarbons (mainly butadiene and isoprene). Natural rubber polymer isoprene: n=1000-3000
Synthetic rubber on an industrial scale for the first time using the method of S.V. Lebedev: It was found that repeated addition of monomeric butadiene-1,3 can occur in the 1,4- and 1,2- positions with the formation of a polymer chain, with double communications. In the presence of metallic sodium.
Rubber is of enormous importance in the national economy.
Polymerization reactions. Diene hydrocarbons have an extremely important feature: they easily enter into polymerization reactions to form rubber-like high-molecular products. Polymerization reactions occur with the addition of molecules to each other in the 1,4- or 1,2-position, as well as with simultaneous addition in the 1,4- and 1,2-positions. This is what a fragment of the formula of the polymerization product of divinyl (1,3 butadiene) looks like if the molecules are added to each other at the 1,4 position.
An analogue of isoprene, chloroprene, easily polymerizes into polychloroprene with the structure:
n (H 2 C=СCl-CH=CH 2) → (-H 2 C-СCl=CH-CH 2 -) 2n
Question 15. Halocarbons–organic compounds formed when one or more cat.H in a hydrocarbon molecule is replaced by a halogen. if, for example, in a molecule of propane, cyclohexane, benzene, only one at.H is replaced by a halogen, then we get a new class of organic compounds - halogenated hydrocarbons, for example: CH3CH2CH2Cl-chloropropane chlorobenzene
Classification: 1 . According to the number of at.H in the molecule, hydrocarbons substituted for halogen are classified into mono-, di-, tri-, tetrahalogen derivatives. ) CC14-carbon tetrachloride (tetra) there are also polyhalogens . 2. Depending on the nature of the at.C, the halogen atoms are connected to the cat; primary R-CH2-Hal, secondary R 2 CH-Ha1 and tertiary R 3 C-Ha1 halogen atoms are distinguished. 3. Depending on relative position Halogen atoms are divided into geminal (when both halogen atts are located at the same at C) - R-CHC1 2 and vicinal (halogen att is found at neighboring at C) - R-CH (C1) - CH2C1 4. Depending on the type and character of the skeletal structure of the org molecules: aliphatic (saturated and unsaturated), cycloaliphotic and aromatic. The name of a halohydrocarbon according to IUPAC nomenclature is based on the name of the longest unbranched chain. Carbon atoms are numbered in such a way that the substituent, which is written first in the name, receives the lower number, and the substituents themselves are listed in alphabetical order. The chains of carbon atoms in the halogen derivatives of alkenes and alkynes are numbered from the end to which the multiple bond is located closest. СНС13-trichloromethane, СН 2 (С1)-СН 2 (С1)-1,2-dichloroethane For some of the simplest halogenated hydrocarbons, names are retained, which are based on the name of the hydrocarbon residue CH 3 Cl - methyl chloride, CH 3 J - methyl iodide, C 2 H 5 Br – ethyl bromide.
The inductive effect (I-effect) is the transfer of the electronic influence of substituents along a chain of σ bonds. This effect is transmitted through a chain of σ-bonds with gradual attenuation and, as a rule, after three to four bonds it no longer appears. The direction of the inductive effect of the substituent is qualitatively assessed by comparing with C-H bond, assuming it is non-polar, and the inductive effect of hydrogen is equal to zero. Electro-withdrawing substituents reduce the electron density in the system of σ-bonds, and they are called electron-withdrawing. Electron-donating substituents increase the electron density in the chain of σ-bonds compared to the hydrogen atom, i.e., they exhibit a +I effect and are electron-donating. These include atoms with low electronegativity (for example, metals), as well as negatively charged atoms or groups that have excess electron density, which they tend to redistribute to neighboring bonds. This effect affects the reactivity of organic molecules, determining both the speed of reaction and the direction of attack of the reagent.


Methods of production: 1. industrial photochemical halogenation (chlorination or bromination) of alkenes under the influence of UV radiation CH4 + C12HC1 + CH3C1-chloromethane. 2. addition of halogens and hydrogen halides through a multiple bond a) CH2=CH-CH3(propene)+Br2CH2(Br)-CH(Br)-CH3-1,2-dibromopropane b) CH2=CH2(ethene)+ HC1CH3CH2C1-chloroethane
Chemical properties: 1.hydrolysis: R-Hal+MeOH (H 2 O) R-OH+MeHal Nucleophilic substitution of halogen, as established, occurs through two mechanisms SN2 - second-order nucleophilic substitution (bimolecular) and SN1 – first order nucleophilic substitution (monomolecular). The order of the reaction corresponds to the number of reagents, the concentration of which determines the reaction rate. 1) SN2 - substitution is most typical for primary alkyl halides. Substitution occurs through the intermediate ( activated complex) in one stage.
2) SN1 – a mechanism typical of tertiary alkyl halides and allylic halides, in which dissociation of the C–Hal bond at the first stage leads to stable carbocations.
Procedures for the elimination of hydrogen halides, nucleophilic substitution in other reactions (see study p. 129
Question16.Comparative characteristics of the chemical properties of alifs and aroma of halohydrocarbons
Question 17. Alcohols and phenols
*
Alcohols are hydroxyl compounds, in which the OH group is never connected to the at.C of the benzene ring. СnH 2 n +1 OH-general formula. Classification: Polyatomic (2 or more OH-groups) and monatomic (one OH-group) are divided into primary, secondary, tertiary.
Isomerism: all types of carbon skeleton, position of the group in the carbon chain (pentanol-2, pentanol-3). Iupak nomenclature: adding to the name of the ancestor u/v suff.-ol. If there are higher phases in the alcohol, then the OH-gr is designated with a prefix (oxy) and the numbering is carried out closer to the end where the OH-gr is located.
Methods of obtaining: 1. hydration of alkenes (i.e. + water) under the influence of t and H3PO4: CH2 = CH2 (ethylene) + NOH CH3-CH2OH-ethanol. 2.hydrolysis of monohalohydrocarbons CH3-CH2Br+ +H2O HBr+CH3-CH2OH (ethanol). 3. oxidation of alkanes (-water) CH3-CH2-CH3+O2 CH3-CH-CH3-propanol-2
Chemical properties: 1.r-ii substitution of atH in OH-gr
2.replacement of OH-gr
3.dehydration
4.oxidation
*
Phenols are hydroxyl compounds, in which the OH group is always connected to the at.C of the benzene ring.
Nomenclature:
Obtaining methods:1. Dow process
2. R-ya Sergeeva
Hi.st.va: 1.r-i substituting OH-gr no
2.r-i substituting at. N in OH-gr
3.P-and substituting atH benz rings
Question 18. Polyhydric alcohols and phenols.
*Polyhydric alcohols are those containing 2 or more functional OH-grs in a molecular composition. Depending on the number of OH-grs, they are divided into two-, three-, and tetraatomic. Diatomic alcohols (glycols) are unstable; at the moment of formation, they lose molecules of water and turn into aldehydes, ketones, and others.
Chemical St. React with alkalis to form salts. For example, ethylene glycol reacts not only with alkali metals, but also with heavy metal hydroxides: 
Glycols with alcohols produce mono-(alcohol esters) and disubstituted products (ethers):
Physical properties: colorless, syrupy liquids with a sweetish taste, highly soluble in water, poorly soluble in organic solvents; have high temperatures boiling. For example, boiling point of ethylene glycol is 198°C, density = 1.11 g/cm 3 ; boiling point of glycerin = 290°C, density = 1.26 g/cm 3 .
Qualitative reaction.
*Phenols are hydroxyl compounds, in which the OH group is always connected to the at.C of the benzene ring.
According to the number of OH groups, all phenols are divided into one-, two-, and triatomic.
Chemical properties 1.r-i substituting OH-gr no
2.r-i substituting at. N in OH-gr
3.P-and substituting atH of the benz-th ring.
a) mutual With alkalis
b) p-ii substitutes. At N of the benzene ring
Physical properties: Most phenols are colorless solids. Phenol melts at t°=41°C. The presence of water in phenol lowers its melting point. A mixture of phenol and water at room temperature is liquid and has a characteristic odor. When heated to 70°C, it dissolves completely. Phenol is an antiseptic, its aqueous solution is used for disinfection and is called carbolic acid.
Qualitative reaction: In aqueous solutions, monoat. phenols react with iron (III) chloride to form complex phenolates, which are purple in color; the color disappears after adding hydrocyanic acid. 6C 6 H 5 OH + FeCl 3 = H 3 + 3HCl
Question 19. Quality. solutions for polyhydric alcohols and primary amino group
Qualitative reaction to polyat. alcohols: Substitution of atH in glycols with heavy metal ions leads to the formation of brightly blue-colored intracomplex chelate-type compounds. Freshly precipitated copper hydroxide with glycols gives:
Qualitative reaction to the primary amino group: Alkylation - When amino acids react with an excess of an alkyl halide, exhaustive alkylation of the amino group occurs and internal salts are formed. 
Question 20. Additions on the carboskil group: addition H, NaHSO 3,HCN,CH 3 MgCl
Question 21. R-and substitution of carbon O 2 in aldehydes and ketones: interaction with PCl 5, NH 3, NH 2 NH 2, NH 2 OH.
Aldehydes-carbonyl compounds, aldehyde content gr.
Ketones are organic substances in whose molecules a carbonyl group is linked to two hydrocarbon radicals.
Question 22. Monocarboxylic acids. Isomerism, nomenclature, methods of production. The structure of the carboxyl group, chemical properties.
Monocarboxylic acids - monobasic carboxylic acids contain one carboxyl group associated with a hydrocarbon radical (saturated, unsaturated, aromatic).
Methods of obtaining: 1. Oxidation of the corresponding aldehydes.
2. Hydrolysis of hymenial trihalocarbons.
3. Hydrolysis of nitriles.
Structure of the carboxyl group: 
Chemical properties: 1. R-ii substitute. At N OH-gr. Interaction with alkalis (neutralization solution).
2. R-ii substitute. OH-gr. a) Formation of esters:
b) image of anhydrides:
c) image of acid halides:
3. Loss of OH-gr
4.R-th by radical R
Question 23. How does the acidity of carboxylic acids depend on the size and character of the radical. How does the presence of acceptor substituents and their position in the mol- le affect? Justify your answer.
Question 24. Functional derivatives of carboxylic acids: salts, esters, anhydrides and acid halides, amides, nitriles. Receiving and St.
Carbon compounds exhibit high reactivity. They come into contact with various things and form various connections, among cats. big meaning. have functional derivatives, i.e. compounds obtained as a result of the carboxyl group.
1. Formation of salts. a) when interacting with metals: 2RCOOH + Mg ® (RCOO)2Mg + H2
b) in reactions with metal hydroxides: 2RCOOH + NaOH ® RCOONa + H2O
2. Formation of esters R"–COOR": (esterification rules) Lower carb esters. set and the simplest monoatoms. alcohols are volatile and colorless. liquid with a characteristic fruity odor. Complex higher carb esters. k-t - colorless. TV things, temp.melt. depends both on the lengths of the carbon chains of the acyl and alcohol residues and on their structure. 
3. Formation of anhydrides. Acetic anhydride is a colorless, transparent, mobile liquid with a pungent odor. .
Acetic anhydride is often used in acylation reactions.
4. Formation of acid halides. Acid halides are highly reactive substances and are widely used in organic synthesis to introduce an acyl group (acylation reaction). 
5. Formation of amides. Formic amide is a liquid, amides of all other compounds are white crystalline substances. Lower amides are highly soluble in water. Water solutions amides give a neutral solution to litmus .
The most important property of amides is their ability to hydrolyze in the presence of acids and alkalis 
6. Formation of nitriles(dehydration of amides)
25. Dicarbonic acids- organ. Compounds containing two carboxyl groups in their molecules. Formula:HOOC-R-COOH. Methods of obtaining. The oxidation of ethylene glycol produces the oxalic acid HO-CH 2 CH 2 -OH
Hydrolysis of dinitriles:N=C-CH 2 CH 2 -C=NNH 2 OC-CH 2 CH 2 -CONH 2 HOOC-CH 2 CH 2 -COOH
Oxidation of cyclic ketones using concentrated nitrogen acid
Chemical propertiesHOOC – R- COOH + CH 3 OHHOOC – R- C-O-CH 3 + H 2 O
HOOC-R-C-OCH 3 + CH 3 OH CH 3 –O-C-R-C-O-CH 3
Heating oxalic and malonic acids with the release of CO 2 and the formation of monocarbonic acids
HOOC-COOH HCOOK + CO 2 HOOC-CH 2 -COOH CH 3 - COOH +CO 2
Malonic acid and its diethyl ether.
Terephthalic acid is a solid, crystalline, high-melting, white substance; obtained by oxidation of n-xylene
Unsaturated compounds contain one or more multiple bonds. They are characterized by all known properties of the carboxyl group and all properties inherent in compounds. Ethylene series. Acrylic compound - 1st member of the homol series is not a precursor, obtained by the oxidation of acrolein
In industry from ethylene oxide and hydrocyanic acid
Mutual influence P– connections and A - position of the radical organ and P- the C=O bond of the carboxyl group leads to the polarization of the first, which causes the orientation of the halogenated hydrocarbons against the Markovnikov rule:
Acrylonitrile is a product of large-scale chemical synthesis; teach by dehydration of hydroxynitrile or catalytic addition of hydrocyanic acid to acetylene at 80 C
Fumaric and maleic acids are isomeric compounds obtained by dehydration of malic acid.
Long-chain higher fatty acids also form geometric isomers. For example, a liquid and oily oleic acid, in the process of slow heating in the presence of catalytic amounts of NO 2, isomerizes into an image of a trans isomer that is already solid under normal conditions - an elaidin acid of the structure:
26. The most important functions of groups in org chemistry. Alcohols are derivatives of hydrocarbons, containing in the molecule one or more hydroxyl groups - OH at saturated carbon atoms. Oxygen-containing organ compounds. By structure they are divided into saturated (alkanols) CH 3 OH methanol, aromatic - phenylmethanol, unsaturated: alkenols CH 2 = CH-CH 2 OH propen-2-ol-1, alkynols HC C-CH 2 OH Propin-2-ol-1 qualities solution for monohydric primary alcohols - copper oxide (calcined copper wire). In this case, alcohols are oxidized to aldehydes, and a layer of reduced copper is formed on the wire.
CH3 - CH2 - OH + CuO =CH3 - COH + Cu + H2O for polyatomic
 The simplest qualitative reaction to alcohols is the oxidation of alcohol with copper oxide. To do this, alcohol vapor is passed over hot copper oxide. Then the resulting aldehyde is captured with fuchsulfurous acid, the solution turns purple:
The simplest qualitative reaction to alcohols is the oxidation of alcohol with copper oxide. To do this, alcohol vapor is passed over hot copper oxide. Then the resulting aldehyde is captured with fuchsulfurous acid, the solution turns purple:
CH 3 -CH 2 -OH + CuO -t-> CH 3 -CHO + Cu + H 2 O Alcohols are identified by the Lucas test - conc. solution of hydrochloric acid and zinc chloride. When a secondary or tertiary alcohol is passed into such a solution, an oily precipitate of the corresponding alkyl chloride is formed:
CH 3 -CHOH-CH 3 + HCl -ZnCl 2 -> CH 3 -CHCl-CH 3 ↓ + H2O
Primary alcohols do not react. Another well-known method is the iodoform test:
CH 3 -CH 2 -OH + 4I 2 + 6NaOH --> CHI 3 ↓ + 5NaI + HCOONa + 5H 2 O Qualitative reactions to polyhydric alcohols.
The most well-known qualitative reaction to polyhydric alcohols is their interaction with copper (II) hydroxide. The hydroxide dissolves and a dark blue chelate complex is formed. Please note that, unlike aldehydes, polyhydric alcohols react with copper (II) hydroxide without heating. For example, when glycerin is added, copper (II) glycerate is formed:  Carbonyl compounds (oxo compounds) - these are hydrocarbon derivatives containing the carbonyl group C=O in the molecule
Carbonyl compounds (oxo compounds) - these are hydrocarbon derivatives containing the carbonyl group C=O in the molecule
Oxocontaining compounds are divided into aldehydes and ketones. Aldehydes are compounds whose molecules contain an aldehyde group associated with the hydrocarbon radical C=O P with an ammonia solution of silver oxide (1) and alkaline solution copper sulfate (2) are high-quality solutions.
CH 3 -CHO + 2OH -t->CH 3 -COOH + 2Ag↓ + 4NH 3 + H 2 O silver mirror reaction
During the reaction, methanoic acid is oxidized to carbonic acid, which decomposes into carbon dioxide and water:
HCOOH + 2OH -t-> CO 2 + 2H 2 O + 4NH 3 + 2Ag↓
In addition to the silver mirror reaction, there is also a reaction with copper (II) hydroxide Cu(OH) 2. To do this, add aldehyde to freshly prepared copper (II) hydroxide and heat the mixture:
CuSO 4 + 2NaOH --> Na 2 SO 4 + Cu(OH) 2 ↓
CH 3 -CHO + 2Cu(OH) 2 -t-> CH 3 -COOH + Cu 2 O↓ + 2H 2 O
Copper oxide (I) Cu 2 O falls out - a red precipitate. Another method for determining aldehydes is the reaction with an alkaline solution of potassium tetraiodomercurate (II), known to us from the previous article as Nessler’s reagent:
CH 3 -CHO + K 2 + 3KOH --> CH 3 -COOK + Hg↓ + 4KI + 2H 2 Carboxylic acids- these are derivatives of hydrocarbons containing the carboxyl group COOH. Formula: Depending on the structure of the hydrocarbon radical, carbonaceous compounds are divided into saturated R = alkyl, unsaturated - derivatives of unsaturated hydrocarbons, aromatic. Nitrogen-containing organic compounds. Amines are derivatives of ammonia (NH 3), in the molecule of which one, two or three hydrogen atoms are replaced by hydrocarbon radicals. Amino acids are derivatives of hydrocarbons, containing amino groups (-NH 2) and carboxy groups Qualitative reactions to amines.
On amines qualitative reactions no (except for aniline). You can prove the presence of an amine by turning litmus blue. If amines cannot be detected, then it is possible to distinguish a primary amine from a secondary one by interaction with nitrous acid HNO2. First you need to prepare it, and then add the amine:
NaNO 2 + HCl -->NaCl + HNO 2 Primary give nitrogen N 2:CH 3 -NH 2 + HNO 2 -->CH 3 -OH + N 2 + H 2 O Secondary - alkylnitrosoamines - substances with a pungent odor (for example, dimethylnitrosoamine ):
CH 3 -NH-CH 3 + HNO 2 --> CH 3 -N(NO)-CH 3 + H 2 O Tertiary amines do not react with HNO 2 under mild conditions. Aniline forms a precipitate when bromine water is added:
C 6 H 5 NH 2 + 3Br 2 --> C6H 2 NH 2 (Br) 3 ↓ + 3HBr
Hydroxy acids
Hydroxy acids Organic carboxylic acids are called
groups. The number of carboxyl groups determines the basicity of the hydroxy acid. By
number of hydroxyls, including those included in carboxyl groups,
determine the atomicity of hydroxy acids.
The simplest hydroxy acids are usually called their natural
source.
For example:
Lactic acid is a monobasic, diatomic acid;
Scheele discovered it in sour milk, which is where it got its name.
HOOC-CH2-CH COOH
OH-apple, dibasic, tribasic
(found in apples).
HOOC-CH-CH COOH
H O OH-tartaric acid, dibasic, tetrahydric
(was isolated from “tartar” - a waste product obtained during the manufacture
and aging of grape wines).
HOOC-CH2-C CH2COOH
Citric acid, tribasic,
tetraatomic, was isolated from lemon leaves.
Very often hydroxy acids are named as hydroxy derivatives
corresponding carboxylic acids. Depending on position
Hydroxy groups in relation to carboxyl groups are distinguished as α-, β-, γ-, etc.
hydroxy acids (hydroxy – according to IUPAC).
Methods of obtaining
1. Hydrolysis of halogen-substituted acids.
This is a convenient way to synthesize α-hydroxy acids due to the availability of α-
halogenated acids.
2. Preparation of aldehydes, ketones(cyanohydrin synthesis, preparation of α-
hydroxy acids).
3. Reduction of oxoacids.
4. Isamino acids.
5. From unsaturated acids.
6. Oxidation of oxyaldehydes (aldols) and glycols.
For example, with silver oxide in an ammonia solution, hydroxy acids are obtained
with different structures depending on the position of the corresponding groups
in the original connection:
7. Oxidation of acids with a tertiary carbon atom located in α-
position to the carboxyl.
8. Hydrolyslactones.
cyclohexanone-caprolactone 6-hydroxyhexanoic acid
Many of the hydroxy acids are obtained specific methods or
extracted from plant and animal products.