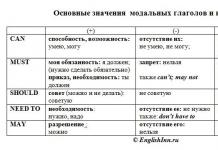
Definition 1
Electrostatics is an extensive branch of electrodynamics that studies and describes electrically charged bodies at rest in a certain system.
In practice, there are two types of electrostatic charges: positive (glass on silk) and negative (hard rubber on wool). The elementary charge is the minimum charge ($e = 1.6 ∙10^( -19)$ C). The charge of any physical body is a multiple of an integer number of elementary charges: $q = Ne$.
Electrification of material bodies is the redistribution of charge between bodies. Methods of electrification: touch, friction and influence.
The law of conservation of electric positive charge - in a closed concept, the algebraic sum of the charges of all elementary particles remains stable and unchanged. $q_1 + q _2 + q _3 + …..+ q_n = const$. The test charge in this case is a point positive charge.
Coulomb's law
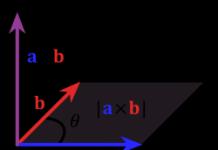
This law was established experimentally in 1785. According to this theory, the force of interaction between two point charges at rest in a medium is always directly proportional to the product of positive moduli and inversely proportional to the square total distance between them.
An electric field is a unique type of matter that interacts between stable electric charges, forms around charges, and affects only charges.
This process of point-like stationary elements completely obeys Newton’s third law, and is considered the result of particles repelling each other with equal force attractions to each other. The relationship between stable electric charges in electrostatics is called the Coulomb interaction.
Coulomb's law is completely fair and accurate for charged material bodies, uniformly charged balls and spheres. In this case, the distances are mainly taken to be the parameters of the centers of spaces. In practice, this law is well and quickly fulfilled if the sizes of charged bodies are much less than the distance between them.
Note 1
Conductors and dielectrics also act in an electric field.
The first represent substances containing free electromagnetic charge carriers. Free movement of electrons can occur inside the conductor. These elements include solutions, metals and various electrolyte melts, ideal gases and plasma.
Dielectrics are substances in which there cannot be free carriers electric charge. The free movement of electrons inside the dielectrics themselves is impossible, since there is no flow through them. electricity. It is these physical particles that have a permeability not equal to the dielectric unit.
Power lines and electrostatics
The lines of force of the initial electric field strength are continuous lines, the tangent points to which in each medium through which they pass completely coincide with the tension axis.
Main characteristics of power lines:
- do not intersect;
- not closed;
- stable;
- the final direction coincides with the direction of the vector;
- start at $+ q$ or at infinity, end at $– q$;
- are formed near charges (where the voltage is greater);
- perpendicular to the surface of the main conductor.
Definition 2
Difference electrical potentials or voltage (Ф or $U$) is the magnitude of the potentials at the initial and final points of the trajectory of a positive charge. The less the potential changes along the path segment, the lower the resulting field strength.
The electric field strength is always directed towards decreasing the initial potential.
Figure 2. Potential energy of a system of electric charges. Author24 - online exchange of student work
Electrical capacity characterizes the ability of any conductor to accumulate the necessary electrical charge on its own surface.
This parameter does not depend on the electric charge, but it can be affected by the geometric dimensions of the conductors, their shapes, location and properties of the medium between the elements.
A capacitor is a universal electrical device that helps quickly accumulate electrical charge for release into a circuit.
Electric field and its intensity
By modern ideas scientists, stable electrical charges do not affect each other directly. Each charged physical body in electrostatics creates environment electric field. This process exerts a force on other charged substances. The main property of the electric field is that it acts on point charges with some force. Thus, the interaction of positively charged particles occurs through the fields that surround the charged elements.
This phenomenon can be studied using the so-called test charge - a small electric charge that does not significantly redistribute the charges being studied. To quantitatively identify the field, a power feature is introduced - the electric field strength.
Tension is a physical indicator that is equal to the ratio of the force with which the field acts on a test charge placed at a given point in the field to the magnitude of the charge itself.
Electric field strength is a vector physical quantity. The direction of the vector in this case coincides at each material point in the surrounding space with the direction of the force acting on the positive charge. The electric field of elements that do not change over time and are stationary is considered electrostatic.
To understand the electric field, lines of force are used, which are drawn in such a way that the direction main axis tension in each system coincided with the direction of the tangent to the point.
Potential difference in electrostatics
The electrostatic field includes one important property: the work done by the forces of all moving particles when moving a point charge from one point in the field to another does not depend on the direction of the trajectory, but is determined solely by the position of the initial and final lines and the charge parameter.
The result of the independence of work from the form of motion of charges is the following statement: the functional of forces electrostatic field when converting charge along any closed trajectory, it is always equal to zero.
Figure 4. Electrostatic field potential. Author24 - online exchange of student work
The property of potentiality of the electrostatic field helps to introduce the concept of potential and internal energy charge. And the physical parameter, equal to the ratio of the potential energy in the field to the value of this charge, is called the constant potential of the electric field.
In many complex tasks electrostatics when determining potentials beyond the reference material point, where the magnitude of the potential energy and the potential itself vanish, it is convenient to use the point at infinity. In this case, the significance of the potential is determined as follows: the potential of the electric field at any point in space is equal to the work that internal forces perform when removing a positive unit charge from a given system to infinity.
Electrical conductivity
Electrical resistance
Electrical impedance
Electrostatics- a section of the study of electricity that studies the interaction of stationary electric charges.
Between of the same name charged bodies, electrostatic (or Coulomb) repulsion occurs, and between different names charged - electrostatic attraction. The phenomenon of repulsion of like charges underlies the creation of an electroscope - a device for detecting electric charges.
Electrostatics is based on Coulomb's law. This law describes the interaction of point electric charges.
Story
The foundation of electrostatics was laid by the work of Coulomb (although ten years before him, the same results, even with even greater accuracy, were obtained by Cavendish. The results of Cavendish’s work were stored in family archive and were published only a hundred years later); the law of electrical interactions discovered by the latter made it possible for Green, Gauss and Poisson to create a mathematically elegant theory. The most essential part of electrostatics is potential theory, created by Green and Gauss. A lot of experimental research on electrostatics was carried out by Rees, whose books in the past constituted the main guide for the study of these phenomena.
The dielectric constant
Finding the value of the dielectric coefficient K of any substance, a coefficient included in almost all formulas that one has to deal with in electrostatics, can be done quite different ways. The most commonly used methods are the following.
1) Comparison of the electrical capacitances of two capacitors having the same size and shape, but in one of which the insulating layer is a layer of air, in the other - a layer of the dielectric being tested.
2) Comparison of attractions between the surfaces of a capacitor, when these surfaces are given a certain potential difference, but in one case there is air between them (attractive force = F 0), in the other case - the test liquid insulator (attractive force = F). The dielectric coefficient is found by the formula:
3) Observations of electric waves (see Electrical vibrations) propagating along wires. According to Maxwell's theory, the speed of propagation of electric waves along wires is expressed by the formula
in which K denotes the dielectric coefficient of the medium surrounding the wire, μ denotes the magnetic permeability of this medium. We can put μ = 1 for the vast majority of bodies, and therefore it turns out
Usually, the lengths of standing electric waves that arise in parts of the same wire that are in the air and in the test dielectric (liquid) are compared. Having determined these lengths λ 0 and λ, we obtain K = λ 0 2 / λ 2. According to Maxwell’s theory, it follows that when an electric field is excited in any insulating substance, special deformations occur inside this substance. Along the induction tubes, the insulating medium is polarized. Electrical displacements arise in it, which can be likened to the movements of positive electricity in the direction of the axes of these tubes, and through each cross section the amount of electricity passing through the tube is equal to
Maxwell's theory makes it possible to find expressions for those internal forces(forces of tension and pressure), which appear in dielectrics when an electric field is excited in them. This question was first considered by Maxwell himself, and later in more detail by Helmholtz. Further development of the theory of this issue and the closely connected theory of electrostriction (that is, a theory that considers phenomena that depend on the occurrence of special voltages in dielectrics when an electric field is excited in them) belongs to the works of Lorberg, Kirchhoff, P. Duhem, N. N. Schiller and some others
Border conditions
Let's finish summary The most significant part of the department of electrostriction is the consideration of the question of refraction of induction tubes. Let us imagine two dielectrics in an electric field, separated from each other by some surface S, with dielectric coefficients K 1 and K 2.
Let at points P 1 and P 2 located infinitely close to the surface S on either side of it, the magnitudes of the potentials are expressed through V 1 and V 2 , and the magnitudes of the forces experienced by a unit of positive electricity placed at these points through F 1 and F 2. Then for a point P lying on the surface S itself, there must be V 1 = V 2,
if ds represents an infinitesimal displacement along the line of intersection of the tangent plane to the surface S at point P with the plane passing through the normal to the surface at this point and through the direction of the electric force in it. On the other hand, it should be
Let us denote by ε 2 the angle made by the force F2 with the normal n2 (inside the second dielectric), and by ε 1 the angle made by the force F 1 with the same normal n 2 Then, using formulas (31) and (30), we find
So, on the surface separating two dielectrics from each other, the electric force undergoes a change in its direction, like a light ray entering from one medium into another. This consequence of the theory is justified by experience.
see also
- Electrostatic discharge
Literature
- Landau, L. D., Lifshits, E. M. Field theory. - 7th edition, revised. - M.: Nauka, 1988. - 512 p. - (“Theoretical Physics”, volume II). - ISBN 5-02-014420-7
- Matveev A. N. Electricity and magnetism. M.: graduate School, 1983.
- Tunnel M.-A. Fundamentals of electromagnetism and the theory of relativity. Per. from fr. M.: Foreign literature, 1962. 488 p.
- Borgman, “Foundations of the doctrine of electrical and magnetic phenomena” (vol. I);
- Maxwell, "Treatise on Electricity and Magnetism" (Vol. I);
- Poincaré, "Electricité et Optique";
- Wiedemann, “Die Lehre von der Elektricität” (vol. I);
Links
- Konstantin Bogdanov. What electrostatics can do // Quantum. - M.: Bureau Quantum, 2010. - No. 2.
Cheat sheet with formulas in physics for the Unified State Exam
and more (may be needed for grades 7, 8, 9, 10 and 11).
First, a picture that can be printed in a compact form.
Mechanics
- Pressure P=F/S
- Density ρ=m/V
- Pressure at liquid depth P=ρ∙g∙h
- Gravity Ft=mg
- 5. Archimedean force Fa=ρ f ∙g∙Vt
- Equation of motion for uniformly accelerated motion
X=X 0 + υ 0 ∙t+(a∙t 2)/2 S=( υ 2 -υ 0 2) /2a S=( υ +υ 0) ∙t /2
- Velocity equation for uniformly accelerated motion υ =υ 0 +a∙t
- Acceleration a=( υ -υ 0)/t
- Circular speed υ =2πR/T
- Centripetal acceleration a= υ 2/R
- Relationship between period and frequency ν=1/T=ω/2π
- Newton's II law F=ma
- Hooke's law Fy=-kx
- Law Universal gravity F=G∙M∙m/R 2
- Weight of a body moving with acceleration a P=m(g+a)
- Weight of a body moving with acceleration а↓ Р=m(g-a)
- Friction force Ftr=µN
- Body momentum p=m υ
- Force impulse Ft=∆p
- Moment of force M=F∙ℓ
- Potential energy of a body raised above the ground Ep=mgh
- Potential energy of an elastically deformed body Ep=kx 2 /2
- Kinetic energy of the body Ek=m υ 2 /2
- Work A=F∙S∙cosα
- Power N=A/t=F∙ υ
- Efficiency η=Ap/Az
- Oscillation period of a mathematical pendulum T=2π√ℓ/g
- Oscillation period of a spring pendulum T=2 π √m/k
- Equation of harmonic vibrations Х=Хmax∙cos ωt
- Relationship between wavelength, its speed and period λ= υ T
Molecular physics and thermodynamics
- Amount of substance ν=N/Na
- Molar mass M=m/ν
- Wed. kin. energy of monatomic gas molecules Ek=3/2∙kT
- Basic MKT equation P=nkT=1/3nm 0 υ 2
- Gay-Lussac's law (isobaric process) V/T =const
- Charles's law (isochoric process) P/T =const
- Relative humidity φ=P/P 0 ∙100%
- Int. energy ideal. monatomic gas U=3/2∙M/µ∙RT
- Gas work A=P∙ΔV
- Boyle–Mariotte law (isothermal process) PV=const
- Amount of heat during heating Q=Cm(T 2 -T 1)
- Amount of heat during melting Q=λm
- Amount of heat during vaporization Q=Lm
- Amount of heat during fuel combustion Q=qm
- Equation of state ideal gas PV=m/M∙RT
- First law of thermodynamics ΔU=A+Q
- Efficiency of heat engines η= (Q 1 - Q 2)/ Q 1
- Efficiency is ideal. engines (Carnot cycle) η= (T 1 - T 2)/ T 1
Electrostatics and electrodynamics - formulas in physics
- Coulomb's law F=k∙q 1 ∙q 2 /R 2
- Electric field strength E=F/q
- Electrical tension point charge field E=k∙q/R 2
- Surface charge density σ = q/S
- Electrical tension fields of an infinite plane E=2πkσ
- Dielectric constant ε=E 0 /E
- Potential energy of interaction. charges W= k∙q 1 q 2 /R
- Potential φ=W/q
- Point charge potential φ=k∙q/R
- Voltage U=A/q
- For a uniform electric field U=E∙d
- Electric capacity C=q/U
- Electric capacity of a flat capacitor C=S∙ ε ∙ε 0 /d
- Energy of a charged capacitor W=qU/2=q²/2С=CU²/2
- Current strength I=q/t
- Conductor resistance R=ρ∙ℓ/S
- Ohm's law for the circuit section I=U/R
- Laws of the last. connections I 1 =I 2 =I, U 1 +U 2 =U, R 1 +R 2 =R
- Laws parallel. conn. U 1 =U 2 =U, I 1 +I 2 =I, 1/R 1 +1/R 2 =1/R
- Electric current power P=I∙U
- Joule-Lenz law Q=I 2 Rt
- Ohm's law for a complete circuit I=ε/(R+r)
- Short circuit current (R=0) I=ε/r
- Magnetic induction vector B=Fmax/ℓ∙I
- Ampere power Fa=IBℓsin α
- Lorentz force Fl=Bqυsin α
- Magnetic flux Ф=BSсos α Ф=LI
- Law electromagnetic induction Ei=ΔФ/Δt
- Induction emf in a moving conductor Ei=Вℓ υ sinα
- Self-induction EMF Esi=-L∙ΔI/Δt
- Energy magnetic field coils Wm=LI 2 /2
- Oscillation period no. circuit T=2π ∙√LC
- Inductive reactance X L =ωL=2πLν
- Capacitance Xc=1/ωC
- Effective current value Id=Imax/√2,
- Effective voltage value Ud=Umax/√2
- Impedance Z=√(Xc-X L) 2 +R 2
Optics
- Law of light refraction n 21 =n 2 /n 1 = υ 1 / υ 2
- Refractive index n 21 =sin α/sin γ
- Thin lens formula 1/F=1/d + 1/f
- Lens optical power D=1/F
- max interference: Δd=kλ,
- min interference: Δd=(2k+1)λ/2
- Differential grid d∙sin φ=k λ
The quantum physics
- Einstein's formula for the photoelectric effect hν=Aout+Ek, Ek=U z e
- Red border of the photoelectric effect ν k = Aout/h
- Photon momentum P=mc=h/ λ=E/s
Physics of the atomic nucleus
Electric charge- This physical quantity, characterizing the ability of particles or bodies to enter into electromagnetic interactions. Electric charge is usually represented by the letters q or Q. In the SI system, electric charge is measured in Coulombs (C). A free charge of 1 C is a gigantic amount of charge, practically not found in nature. Typically, you will have to deal with microcoulombs (1 µC = 10 -6 C), nanocoulombs (1 nC = 10 -9 C) and picoculombs (1 pC = 10 -12 C). Electric charge has the following properties:
1. Electric charge is a type of matter.
2. The electric charge does not depend on the movement of the particle and its speed.
3. Charges can be transferred (for example, by direct contact) from one body to another. Unlike body mass, electric charge is not an integral characteristic of a given body. The same body under different conditions can have a different charge.
4. There are two types of electric charges, conventionally called positive And negative.
5. All charges interact with each other. In this case, like charges repel, unlike charges attract. The forces of interaction between charges are central, that is, they lie on a straight line connecting the centers of the charges.
6. There is a minimum possible (modulo) electric charge, called elementary charge. Its meaning:
e= 1.602177·10 –19 C ≈ 1.6·10 –19 C.
The electric charge of any body is always a multiple of the elementary charge:
Where: N– an integer. Please note that it is impossible for a charge equal to 0.5 to exist e; 1,7e; 22,7e and so on. Physical quantities that can only take a discrete (not continuous) series of values are called quantized. Elementary charge e is a quantum (smallest portion) of electric charge.
In an isolated system, the algebraic sum of the charges of all bodies remains constant:
The law of conservation of electric charge states that in a closed system of bodies processes of creation or disappearance of charges of only one sign cannot be observed. It also follows from the law of conservation of charge that if two bodies of the same size and shape having charges q 1 and q 2 (it doesn’t matter at all what sign the charges are), bring into contact, and then separate again, then the charge of each of the bodies will become equal:
From a modern point of view, charge carriers are elementary particles. All ordinary bodies are made up of atoms, which contain positively charged protons, negatively charged electrons and neutral particles - neutrons. Protons and neutrons are part of atomic nuclei, electrons form the electron shell of atoms. The electric charges of a proton and an electron are exactly the same in magnitude and equal to the elementary (that is, the minimum possible) charge e.
In a neutral atom, the number of protons in the nucleus is equal to the number of electrons in the shell. This number is called the atomic number. An atom of a given substance may lose one or more electrons, or gain an extra electron. In these cases, the neutral atom turns into a positively or negatively charged ion. Please note that positive protons are part of the nucleus of an atom, so their number can only change during nuclear reactions. It is obvious that when bodies are electrified nuclear reactions not happening. Therefore, in any electrical phenomena, the number of protons does not change, only the number of electrons changes. Thus, imparting a negative charge to a body means transferring extra electrons to it. And the message of a positive charge, contrary to a common mistake, does not mean the addition of protons, but the subtraction of electrons. Charge can be transferred from one body to another only in portions containing an integer number of electrons.
Sometimes in problems the electric charge is distributed over a certain body. To describe this distribution, the following quantities are introduced:
1. Linear charge density. Used to describe the distribution of charge along the filament:
Where: L– thread length. Measured in C/m.
2. Surface charge density. Used to describe the distribution of charge over the surface of a body:
Where: S– body surface area. Measured in C/m2.
3. Volume charge density. Used to describe the distribution of charge over the volume of a body:
Where: V– body volume. Measured in C/m3.
Please note that electron mass is equal to:
m e= 9.11∙10 –31 kg.
Coulomb's law
Point charge called a charged body, the dimensions of which can be neglected in the conditions of this problem. Based on numerous experiments, Coulomb established the following law:
The interaction forces between stationary point charges are directly proportional to the product of the charge moduli and inversely proportional to the square of the distance between them:

Where: ε – dielectric constant of a medium is a dimensionless physical quantity that shows how many times the force of electrostatic interaction in a given medium will be less than in a vacuum (that is, how many times the medium weakens the interaction). Here k– coefficient in Coulomb’s law, a value that determines the numerical value of the force of interaction between charges. In the SI system its value is taken equal to:
k= 9∙10 9 m/F.
The forces of interaction between point fixed charges obey Newton's third law, and are forces of repulsion from each other when the charges have the same signs and forces of attraction to each other when different signs. The interaction of stationary electric charges is called electrostatic or Coulomb interaction. The branch of electrodynamics that studies the Coulomb interaction is called electrostatics.
Coulomb's law is valid for point charged bodies, uniformly charged spheres and balls. In this case, for distances r take the distance between the centers of spheres or balls. In practice, Coulomb's law is well satisfied if the sizes of charged bodies are much smaller than the distance between them. Coefficient k in the SI system it is sometimes written as:
![]()
Where: ε 0 = 8.85∙10 –12 F/m – electrical constant.
Experience shows that the forces of Coulomb interaction obey the principle of superposition: if a charged body interacts simultaneously with several charged bodies, then the resulting force acting on this body is equal to vector sum forces acting on this body from all other charged bodies.
Remember also two important definitions:
Conductors– substances containing free electric charge carriers. Inside a conductor, free movement of electrons - charge carriers - is possible (electric current can flow through the conductors). Conductors include metals, solutions and melts of electrolytes, ionized gases, and plasma.
Dielectrics (insulators)– substances in which there are no free charge carriers. The free movement of electrons inside dielectrics is impossible (electric current cannot flow through them). It is dielectrics that have a certain dielectric constant, not equal to unity. ε .
For the dielectric constant of a substance, the following is true (about what an electric field is just below):

Electric field and its intensity
According to modern concepts, electric charges do not act on each other directly. Each charged body creates in the surrounding space electric field. This field exerts a force on other charged bodies. The main property of the electric field is the effect on electric charges with some force. Thus, the interaction of charged bodies is carried out not by their direct influence on each other, but through the electric fields surrounding the charged bodies.
The electric field surrounding a charged body can be studied using a so-called test charge - a small point charge that does not introduce a noticeable redistribution of the charges being studied. To quantitatively determine the electric field, a force characteristic is introduced - electric field strength E.
Electric field strength is a physical quantity equal to the ratio of the force with which the field acts on a test charge placed in this point fields, to the magnitude of this charge:

Electric field strength is a vector physical quantity. The direction of the tension vector coincides at each point in space with the direction of the force acting on the positive test charge. The electric field of stationary charges that do not change over time is called electrostatic.
To visually represent the electric field, use power lines. These lines are drawn so that the direction of the tension vector at each point coincides with the direction of the tangent to the line of force. Field lines have the following properties.
- Electrostatic field lines never intersect.
- The electrostatic field lines are always directed from positive to negative charges.
- When depicting an electric field using field lines, their density should be proportional to the magnitude of the field strength vector.
- Lines of force begin at a positive charge, or infinity, and end at a negative charge, or infinity. The greater the tension, the greater the density of the lines.
- At a given point in space, only one line of force can pass, because The electric field strength at a given point in space is specified uniquely.
An electric field is called uniform if the intensity vector is the same at all points of the field. For example, a uniform field is created by a flat capacitor - two plates charged with a charge of equal magnitude and opposite sign, separated by a dielectric layer, and the distance between the plates is much less than the size of the plates.
At all points uniform field per charge q, introduced into a uniform field with intensity E, a force of equal magnitude and direction acts, equal to F = Eq. Moreover, if the charge q positive, then the direction of the force coincides with the direction of the tension vector, and if the charge is negative, then the force and tension vectors are oppositely directed.
Positive and negative point charges are shown in the figure:

Superposition principle
If an electric field created by several charged bodies is studied using a test charge, then the resulting force turns out to be equal to the geometric sum of the forces acting on the test charge from each charged body separately. Consequently, the electric field strength created by a system of charges at a given point in space is equal to the vector sum of the electric field strengths created at the same point by charges separately:
![]()
This property of the electric field means that the field obeys superposition principle. In accordance with Coulomb's law, the strength of the electrostatic field created by a point charge Q on distance r from it, is equal in modulus:

This field is called Coulomb field. In a Coulomb field, the direction of the intensity vector depends on the sign of the charge Q: If Q> 0, then the voltage vector is directed away from the charge, if Q < 0, то вектор напряженности направлен к заряду. Величина напряжённости зависит от величины заряда, среды, в которой находится заряд, и уменьшается с увеличением расстояния.
The electric field strength created by a charged plane near its surface:
So, if the problem requires determining the field strength of a system of charges, then we must proceed as follows algorithm:
- Draw a picture.
- Draw the field strength of each charge separately in the right point. Remember that tension is directed towards a negative charge and away from a positive charge.
- Calculate each of the tensions using the appropriate formula.
- Add the stress vectors geometrically (i.e. vectorially).
Potential energy of charge interaction
Electric charges interact with each other and with the electric field. Any interaction is described by potential energy. Potential energy of interaction of two point electric charges calculated by the formula:
Please note that the charges have no modules. For unlike charges, the interaction energy has negative meaning. The same formula is valid for the interaction energy of uniformly charged spheres and balls. As usual, in this case the distance r is measured between the centers of the balls or spheres. If there are not two, but more charges, then the energy of their interaction should be calculated as follows: divide the system of charges into all possible pairs, calculate the interaction energy of each pair and sum up all the energies for all pairs.
Problems on this topic are solved, as well as problems on the conservation law mechanical energy: first the initial energy of interaction is found, then the final one. If the problem asks you to find the work done by moving charges, then it will be equal to the difference between the initial and final total energy of interaction of charges. Interaction energy can also be converted into kinetic energy or other types of energy. If the bodies are at a very large distance, then the energy of their interaction is assumed to be equal to 0.
Please note: if the problem requires finding the minimum or maximum distance between bodies (particles) when moving, then this condition will be met at that moment in time when the particles move in one direction at the same speed. Therefore, the solution must begin by writing down the law of conservation of momentum, from which this identical speed is found. And then we should write the law of conservation of energy, taking into account the kinetic energy of particles in the second case.
Potential. Potential difference. Voltage
The electrostatic field has an important property: the work of the electrostatic field forces when moving a charge from one point in the field to another does not depend on the shape of the trajectory, but is determined only by the position of the starting and ending points and the magnitude of the charge.
A consequence of the independence of work from the shape of the trajectory is the following statement: the work of electrostatic field forces when moving a charge along any closed trajectory is equal to zero.
The property of potentiality (independence of work from the shape of the trajectory) of the electrostatic field allows us to introduce the concept of potential energy of a charge in an electric field. And a physical quantity equal to the ratio of the potential energy of an electric charge in an electrostatic field to the magnitude of this charge is called potential φ electric field:
Potential φ is the energy characteristic of the electrostatic field. In the International System of Units (SI), the unit of potential (and therefore potential difference, i.e. voltage) is the volt [V]. Potential is a scalar quantity.
In many problems of electrostatics, when calculating potentials, it is convenient to take the point at infinity as the reference point where the values of potential energy and potential vanish. In this case, the concept of potential can be defined as follows: the field potential at a given point in space is equal to the work done by electric forces when removing a single positive charge from a given point to infinity.
Recalling the formula for the potential energy of interaction of two point charges and dividing it by the value of one of the charges in accordance with the definition of potential, we obtain that potential φ point charge fields Q on distance r from it relative to a point at infinity is calculated as follows:
The potential calculated using this formula can be positive or negative depending on the sign of the charge that created it. The same formula expresses the field potential of a uniformly charged ball (or sphere) at r ≥ R(outside the ball or sphere), where R is the radius of the ball, and the distance r measured from the center of the ball.
To visually represent the electric field, along with lines of force, use equipotential surfaces. A surface at all points of which the electric field potential has the same values is called an equipotential surface or a surface of equal potential. Electric field lines are always perpendicular to equipotential surfaces. The equipotential surfaces of the Coulomb field of a point charge are concentric spheres.
Electrical voltage it's just a potential difference, i.e. The definition of electrical voltage can be given by the formula:
![]()
In a uniform electric field there is a relationship between field strength and voltage:
Electric field work can be calculated as the difference between the initial and final potential energy of a system of charges:
The work of the electric field in the general case can also be calculated using one of the formulas:
In a uniform field, when a charge moves along its field lines, the work of the field can also be calculated using the following formula:
In these formulas:
- φ – electric field potential.
- ∆φ – potential difference.
- W– potential energy of a charge in an external electric field.
- A– the work of the electric field to move the charge (charges).
- q– a charge that moves in an external electric field.
- U- voltage.
- E– electric field strength.
- d or ∆ l– the distance to which the charge is moved along the lines of force.
In all previous formulas we were talking specifically about the work of the electrostatic field, but if the problem says that “work must be done,” or we're talking about About work external forces", then this work should be considered the same as the field work, but with the opposite sign.
Potential superposition principle
From the principle of superposition of field strengths created by electric charges, the principle of superposition for potentials follows (in this case, the sign of the field potential depends on the sign of the charge that created the field):
Notice how much easier it is to apply the principle of superposition of potential than of tension. Potential is a scalar quantity that has no direction. Adding potentials is simply adding up numerical values.
Electrical capacity. Flat capacitor
When imparting a charge to a conductor, there is always a certain limit beyond which it will not be possible to charge the body. To characterize the body’s ability to accumulate electrical charge, the concept is introduced electrical capacitance. The capacitance of an isolated conductor is the ratio of its charge to potential:
In the SI system, capacitance is measured in Farads [F]. 1 Farad is an extremely large capacitance. For comparison, the capacity of only globe significantly less than one farad. The capacitance of a conductor depends neither on its charge nor on the potential of the body. Similarly, density does not depend on either the mass or volume of the body. Capacity depends only on the shape of the body, its size and the properties of its environment.
Electric capacity system of two conductors is a physical quantity defined as the ratio of charge q one of the conductors to the potential difference Δ φ between them:
![]()
The magnitude of the electrical capacitance of conductors depends on the shape and size of the conductors and on the properties of the dielectric separating the conductors. There are configurations of conductors in which the electric field is concentrated (localized) only in a certain region of space. Such systems are called capacitors, and the conductors that make up the capacitor are called linings.
The simplest capacitor is a system of two flat conducting plates located parallel to each other at a small distance compared to the size of the plates and separated by a dielectric layer. Such a capacitor is called flat. The electric field of a parallel-plate capacitor is mainly localized between the plates.
Each of the charged plates of a flat capacitor creates an electric field near its surface, the modulus of which is expressed by the relationship already given above. Then the modulus of the final field strength inside the capacitor created by the two plates is equal to:

Outside the capacitor, the electric fields of the two plates are directed in different directions, and therefore the resulting electrostatic field E= 0. can be calculated using the formula:
Thus, the electrical capacity of a flat capacitor is directly proportional to the area of the plates (plates) and inversely proportional to the distance between them. If the space between the plates is filled with a dielectric, the capacitance of the capacitor increases by ε once. note that S in this formula there is the area of only one capacitor plate. When they talk about the “plating area” in a problem, they mean exactly this value. You never need to multiply or divide it by 2.
Once again we present the formula for capacitor charge. The charge of a capacitor is understood only as the charge on its positive plate:
The force of attraction between the capacitor plates. The force acting on each plate is determined not by the total field of the capacitor, but by the field created by the opposite plate (the plate does not act on itself). The strength of this field is equal to half the strength of the total field, and the force of interaction between the plates is:
Capacitor energy. It is also called the energy of the electric field inside the capacitor. Experience shows that a charged capacitor contains a reserve of energy. The energy of a charged capacitor is equal to the work of external forces that must be expended to charge the capacitor. There are three equivalent forms of writing the formula for the energy of a capacitor (they follow one from the other if we use the relation q = C.U.):
![]()
Pay special attention to the phrase: “The capacitor is connected to the source.” This means that the voltage across the capacitor does not change. And the phrase “The capacitor was charged and disconnected from the source” means that the charge of the capacitor will not change.
Electric field energy
Electrical energy should be considered as potential energy stored in a charged capacitor. According to modern ideas, Electric Energy of the capacitor is localized in the space between the plates of the capacitor, that is, in the electric field. Therefore it is called electric field energy. The energy of charged bodies is concentrated in space in which there is an electric field, i.e. we can talk about the energy of the electric field. For example, a capacitor's energy is concentrated in the space between its plates. Thus, it makes sense to introduce a new physical characteristics– volumetric energy density of the electric field. Using a flat capacitor as an example, we can obtain the following formula for the volumetric energy density (or the energy per unit volume of the electric field):
Capacitor connections
Parallel connection of capacitors– to increase capacity. The capacitors are connected by similarly charged plates, as if increasing the area of the equally charged plates. The voltage on all capacitors is the same, the total charge is equal to the sum of the charges of each capacitor, and the total capacitance is also equal to the sum of the capacitances of all capacitors connected in parallel. Let's write down the formulas for parallel connection of capacitors:

At series connection of capacitors the total capacity of a capacitor bank is always less than the capacity of the smallest capacitor included in the battery. A series connection is used to increase the breakdown voltage of the capacitors. Let's write down the formulas for connecting capacitors in series. The total capacitance of series-connected capacitors is found from the relationship:
![]()
From the law of conservation of charge it follows that the charges on adjacent plates are equal:
The voltage is equal to the sum of the voltages on the individual capacitors.
For two capacitors connected in series, the formula above will give us the following expression for the total capacitance:
For N identical series-connected capacitors:
Conductive sphere
The field strength inside a charged conductor is zero. Otherwise, an electric force would act on the free charges inside the conductor, which would force these charges to move inside the conductor. This movement, in turn, would lead to heating of the charged conductor, which actually does not happen.
The fact that there is no electric field inside the conductor can be understood in another way: if there was one, then the charged particles would again move, and they would move exactly in such a way as to reduce this field to zero with their own field, because in fact, they would not want to move, because every system strives for balance. Sooner or later, all moving charges would stop exactly in that place so that the field inside the conductor would become zero.
On the surface of the conductor, the electric field strength is maximum. The magnitude of the electric field strength of a charged ball outside its boundaries decreases with distance from the conductor and is calculated using a formula similar to the formula for the field strength of a point charge, in which distances are measured from the center of the ball.
Since the field strength inside a charged conductor is zero, the potential at all points inside and on the surface of the conductor is the same (only in this case the potential difference, and therefore the voltage, is zero). The potential inside a charged ball is equal to the potential on the surface. The potential outside the ball is calculated using a formula similar to the formulas for the potential of a point charge, in which distances are measured from the center of the ball.
Radius R:
If the ball is surrounded by a dielectric, then:
Properties of a conductor in an electric field
- Inside a conductor, the field strength is always zero.
- The potential inside the conductor is the same at all points and is equal to the potential of the surface of the conductor. When they say in a problem that “the conductor is charged to a potential ... V,” they mean precisely the surface potential.
- Outside the conductor near its surface, the field strength is always perpendicular to the surface.
- If a charge is given to a conductor, then it will all be distributed over a very thin layer near the surface of the conductor (usually they say that the entire charge of the conductor is distributed on its surface). This is easily explained: the fact is that when imparting a charge to a body, we transfer to it charge carriers of the same sign, i.e. like charges that repel each other. This means they will try to run away from each other to the maximum possible distance, i.e. accumulate at the very edges of the conductor. As a result, if the core is removed from a conductor, its electrostatic properties will not change in any way.
- Outside the conductor, the more curved the surface of the conductor, the greater the field strength. The maximum value of tension is achieved near the edges and sharp breaks in the surface of the conductor.
Notes on solving complex problems
1. Grounding something means the connection of a conductor of this object with the Earth. In this case, the potentials of the Earth and the existing object are equalized, and the charges necessary for this move along the conductor from the Earth to the object or vice versa. In this case, it is necessary to take into account several factors that follow from the fact that the Earth is disproportionately larger than any object located on it:
- The total charge of the Earth is conventionally zero, so its potential is also zero, and it will remain zero after the object connects with the Earth. In a word, to ground means to reset the potential of an object.
- To reset the potential (and therefore the object’s own charge, which could previously have been either positive or negative), the object will have to either accept or give to the Earth some (perhaps even a very large) charge, and the Earth will always be able to provide this possibility.
2. Let us repeat once again: the distance between repelling bodies is minimal at the moment when their velocities become equal in magnitude and directed in the same direction ( relative speed charges is zero). At this moment, the potential energy of interaction of charges is maximum. The distance between attracting bodies is maximum, also at the moment of equality of velocities directed in one direction.
3. If the problem involves a system consisting of a large number of charges, then it is necessary to consider and describe the forces acting on a charge that is not located at the center of symmetry.
Successful, diligent and responsible implementation of these three points, as well as responsible study of the final training tests, will allow you to show an excellent result at the CT, the maximum of what you are capable of.
Found a mistake?
If you think you have found an error in educational materials, then please write about it on email(). In the letter, indicate the subject (physics or mathematics), the name or number of the topic or test, the number of the problem, or the place in the text (page) where, in your opinion, there is an error. Also describe what the suspected error is. Your letter will not go unnoticed, the error will either be corrected, or you will be explained why it is not an error.
In electrostatics, one of the fundamental ones is Coulomb's law. It is used in physics to determine the force of interaction between two stationary point charges or the distance between them. This is a fundamental law of nature that does not depend on any other laws. Then the shape of the real body does not affect the magnitude of the forces. In this article we will tell in simple language Coulomb's law and its application in practice.
History of discovery
Sh.O. Coulomb in 1785 was the first to experimentally prove the interactions described by the law. In his experiments he used special torsion balances. However, back in 1773, Cavendish proved, using the example of a spherical capacitor, that there is no electric field inside the sphere. This indicated that electrostatic forces vary depending on the distance between the bodies. To be more precise - the square of the distance. His research was not published then. Historically, this discovery was named after Coulomb, and the quantity in which charge is measured has a similar name.
Formulation
The definition of Coulomb's law states: In a vacuumF interaction of two charged bodies is directly proportional to the product of their moduli and inversely proportional to the square of the distance between them.
It sounds short, but may not be clear to everyone. In simple words: The more charge the bodies have and the closer they are to each other, the greater the force.
And vice versa: If you increase the distance between the charges, the force will become less.
The formula for Coulomb's rule looks like this:
Designation of letters: q - charge value, r - distance between them, k - coefficient, depends on the chosen system of units.
The charge value q can be conditionally positive or conditionally negative. This division is very arbitrary. When bodies come into contact, it can be transmitted from one to another. It follows that the same body can have a charge of different magnitude and sign. A point charge is a charge or body whose dimensions are much smaller than the distance of possible interaction.
It is worth considering that the environment in which the charges are located affects the F interaction. Since it is almost equal in air and vacuum, Coulomb’s discovery is applicable only for these media; this is one of the conditions for the use of this type of formula. As already mentioned, in the SI system the unit of measurement of charge is Coulomb, abbreviated Cl. It characterizes the amount of electricity per unit time. It is derived from the SI base units.
1 C = 1 A*1 s
It is worth noting that the dimension of 1 C is redundant. Due to the fact that carriers repel each other, it is difficult to contain them in a small body, although the 1A current itself is small if it flows in a conductor. For example, in the same 100 W incandescent lamp a current of 0.5 A flows, and in an electric heater it flows more than 10 A. Such a force (1 C) is approximately equal to the 1 ton mass acting on a body from the side of the globe.
You may have noticed that the formula is almost the same as in gravitational interaction, only if masses appear in Newtonian mechanics, then charges appear in electrostatics.
Coulomb formula for a dielectric medium
The coefficient, taking into account the values of the SI system, is determined in N 2 * m 2 / Cl 2. It is equal to:

In many textbooks, this coefficient can be found in the form of a fraction:

Here E 0 = 8.85*10-12 C2/N*m2 is the electrical constant. For a dielectric, E is added - the dielectric constant of the medium, then Coulomb's law can be used to calculate the forces of interaction of charges for vacuum and medium.
Taking into account the influence of the dielectric, it has the form:

From this we see that the introduction of a dielectric between the bodies reduces the force F.
How are the forces directed?
Charges interact with each other depending on their polarity - like charges repel, and unlike (opposite) charges attract.


By the way, this is the main difference from a similar law of gravitational interaction, where bodies always attract. The forces are directed along the line drawn between them, called the radius vector. In physics it is denoted as r 12 and as the radius vector from the first to the second charge and vice versa. The forces are directed from the center of the charge to the opposite charge along this line, if the charges are opposite, and in reverse side, if they are of the same name (two positive or two negative). In vector form:

The force applied to the first charge by the second is denoted as F 12. Then, in vector form, Coulomb’s law looks like this:

To determine the force applied to the second charge, the designations F 21 and R 21 are used.
If the body has a complex shape and is large enough that at a given distance it cannot be considered a point charge, then it is divided into small sections and each section is considered a point charge. After geometrically adding all the resulting vectors, the resulting force is obtained. Atoms and molecules interact with each other according to the same law.
Application in practice
Coulomb's work is very important in electrostatics; in practice, it is used in a number of inventions and devices. A striking example You can select a lightning rod. With its help, they protect buildings and electrical installations from thunderstorms, thereby preventing fire and equipment failure. When it rains with a thunderstorm, an induced charge of large magnitude appears on the ground, they are attracted towards the cloud. It turns out that a large electric field appears on the surface of the earth. Near the tip of the lightning rod it is larger, as a result of which a corona discharge is ignited from the tip (from the ground, through the lightning rod to the cloud). The charge from the ground is attracted to the opposite charge of the cloud, according to Coulomb's law. The air is ionized, and the electric field strength decreases near the end of the lightning rod. Thus, charges do not accumulate on the building, in which case the likelihood of a lightning strike is low. If a strike does occur on the building, then all the energy will go into the ground through the lightning rod.
In serious scientific research They use the greatest construction of the 21st century - a particle accelerator. In it, the electric field does work to increase the energy of the particle. Considering these processes from the point of view of the influence of a group of charges on a point charge, then all the relations of the law turn out to be valid.
Useful