Many natural phenomena indicate the chaotic movement of microparticles, molecules and atoms of matter. The higher the temperature of the substance, the more intense this movement. Therefore, the heat of a body is a reflection of the random movement of its constituent molecules and atoms.
Proof that all atoms and molecules of a substance are in constant and random motion can be diffusion - the interpenetration of particles of one substance into another (see Fig. 20a). Thus, the smell quickly spreads throughout the room even in the absence of air movement. A drop of ink quickly turns the entire glass of water uniformly black, although it would seem that gravity should help color the glass only in the top-to-bottom direction. Diffusion can also be detected in solids if they are pressed tightly together and left to long time. The phenomenon of diffusion demonstrates that microparticles of a substance are capable of spontaneous movement in all directions. This movement of microparticles of a substance, as well as its molecules and atoms, is called thermal movement.
Obviously, all the water molecules in the glass are moving even if there is no drop of ink in it. Simply, the diffusion of ink makes the thermal movement of molecules noticeable. Another phenomenon that makes it possible to observe thermal motion and even evaluate its characteristics can be Brownian motion, which refers to the chaotic movement of any smallest particles in a completely calm liquid visible through a microscope. It was named Brownian in honor of the English botanist R. Brown, who in 1827, examining pollen spores of one of the plants suspended in water through a microscope, discovered that they were continuously and chaotically moving.
Brown's observation was confirmed by many other scientists. It turned out that Brownian motion is not associated either with flows in the liquid or with its gradual evaporation. The smallest particles (they were also called Brownian) behaved as if they were alive, and this “dance” of particles accelerated with heating of the liquid and with a decrease in particle size and, conversely, slowed down when replacing water with a more viscous medium. Brownian motion was especially noticeable when it was observed in gas, for example, by following particles of smoke or droplets of fog in the air. This amazing phenomenon never stopped, and it could be observed for as long as desired.
An explanation of Brownian motion was given only in the last quarter of the 19th century, when it became obvious to many scientists that the motion of a Brownian particle is caused by random impacts of molecules of the medium (liquid or gas) undergoing thermal motion (see Fig. 20b). On average, the molecules of the medium influence the Brownian particle from all sides from equal strength However, these impacts never exactly cancel each other out, and as a result, the speed of the Brownian particle varies randomly in magnitude and direction. Therefore, the Brownian particle moves along a zigzag path. Moreover, the smaller the size and mass of a Brownian particle, the more noticeable its movement becomes.
In 1905, A. Einstein created the theory of Brownian motion, believing that at any given moment in time the acceleration of a Brownian particle depends on the number of collisions with molecules of the medium, which means it depends on the number of molecules per unit volume of the medium, i.e. from Avogadro's number. Einstein derived a formula by which it was possible to calculate how the mean square of the displacement of a Brownian particle changes over time, if you know the temperature of the medium, its viscosity, the size of the particle and Avogadro's number, which was still unknown at that time. The validity of this Einstein theory was confirmed experimentally by J. Perrin, who was the first to obtain the value of Avogadro's number. Thus, the analysis of Brownian motion laid the foundations of the modern molecular kinetic theory of the structure of matter.
Review questions:
· What is diffusion, and how is it related to the thermal movement of molecules?
· What is called Brownian motion, and is it thermal?
· How does the nature of Brownian motion change when heated?
Rice. 20. (a) – the upper part shows molecules of two different gases separated by a partition, which is removed (see lower part), after which diffusion begins; (b) in the lower left part there is a schematic representation of a Brownian particle (blue), surrounded by molecules of the medium, collisions with which cause the particle to move (see three trajectories of the particle).
§ 21. INTERMOLECULAR FORCES: STRUCTURE OF GASEOUS, LIQUID AND SOLID BODIES
We are accustomed to the fact that liquid can be poured from one vessel to another, and gas quickly fills the entire volume provided to it. Water can only flow along the riverbed, and the air above it knows no boundaries. If the gas did not try to occupy all the space around us, we would suffocate, because... The carbon dioxide we exhale would accumulate near us, preventing us from taking a breath of fresh air. Yes, and the cars would soon stop for the same reason, because... They also need oxygen to burn fuel.
Why does a gas, unlike a liquid, fill the entire volume provided to it? Between all molecules there are intermolecular forces of attraction, the magnitude of which decreases very quickly as the molecules move away from each other, and therefore at a distance equal to several diameters of the molecules, they do not interact at all. It is easy to show that the distance between neighboring gas molecules is many times greater than that of a liquid. Using formula (19.3) and knowing the air density (r=1.29 kg/m3) at atmospheric pressure and its molar mass(M=0.029 kg/mol), we can calculate the average distance between air molecules, which will be equal to 6.1.10-9 m, which is twenty times greater than the distance between water molecules.
Thus, between liquid molecules located almost close to each other, attractive forces act, preventing these molecules from scattering in different directions. On the contrary, the insignificant forces of attraction between gas molecules are not able to hold them together, and therefore gases can expand, filling the entire volume provided to them. The existence of intermolecular attractive forces can be verified by performing a simple experiment - pressing two lead bars against each other. If the contact surfaces are sufficiently smooth, the bars will stick together and will be difficult to separate.
However, intermolecular attractive forces alone cannot explain all the differences between the properties of gaseous, liquid and solid substances. Why, for example, is it very difficult to reduce the volume of a liquid or solid, but to compress balloon relatively easy? This is explained by the fact that between molecules there are not only attractive forces, but also intermolecular repulsive forces, which act when the electronic shells of the atoms of neighboring molecules begin to overlap. It is these repulsive forces that prevent one molecule from penetrating into a volume already occupied by another molecule.
When a liquid or solid body is not acted upon by external forces, the distance between their molecules is such (see r0 in Fig. 21a) at which the resultant forces of attraction and repulsion are equal to zero. If you try to reduce the volume of a body, the distance between the molecules decreases, and the resulting increased repulsive forces begin to act from the side of the compressed body. On the contrary, when a body is stretched, the elastic forces that arise are associated with a relative increase in the forces of attraction, because when molecules move away from each other, the repulsive forces fall much faster than the attractive forces (see Fig. 21a).
Gas molecules are located at distances tens of times greater than their sizes, as a result of which these molecules do not interact with each other, and therefore gases are much more easily compressed than liquids and solids. Gases do not have any specific structure and are a collection of moving and colliding molecules (see Fig. 21b).
A liquid is a collection of molecules that are almost closely adjacent to each other (see Fig. 21c). Thermal motion allows a liquid molecule to change its neighbors from time to time, jumping from one place to another. This explains the fluidity of liquids.
Atoms and molecules of solids are deprived of the ability to change their neighbors, and their thermal motion is only small fluctuations relative to the position of neighboring atoms or molecules (see Fig. 21d). The interaction between atoms can lead to the fact that a solid becomes a crystal, and the atoms in it occupy positions at the sites of the crystal lattice. Since the molecules of solid bodies do not move relative to their neighbors, these bodies retain their shape.
Review questions:
· Why don't gas molecules attract each other?
· What properties of bodies determine the intermolecular forces of repulsion and attraction?
How do you explain the fluidity of a liquid?
· Why do all solids retain their shape?
§ 22. IDEAL GAS. BASIC EQUATION OF THE MOLECULAR-KINETIC THEORY OF GASES.
liquids, amorphous and crystalline bodies
gases and liquids
gases, liquids and crystalline solids
approximately equal to the diameter of the molecule
smaller than the diameter of the molecule
approximately 10 times the diameter of the molecule
depends on gas temperature
liquids
crystalline bodies
amorphous bodies
only gas structure models
only models of the structure of amorphous bodies
models of the structure of gases and liquids
models of the structure of gases, liquids and solids
the distance between molecules increases
molecules begin to attract each other
orderliness in the arrangement of molecules increases
the distance between molecules decreases
hasn't changed
increased 5 times
decreased by 5 times
increased by the root of five
The distances between molecules are comparable to the sizes of molecules (at normal conditions) For
In gases under normal conditions, the average distance between molecules is
The least order in the arrangement of particles is characteristic of
The distance between neighboring particles of matter is on average many times greater than the size of the particles themselves. This statement corresponds to the model
During the transition of water from liquid state into crystalline
At constant pressure, the concentration of gas molecules increased 5 times, but its mass did not change. Average kinetic energy forward movement gas molecules
The table shows the melting and boiling points of some substances:
substance | Boiling temperature | substance | Melting temperature |
naphthalene |
Choose the correct statement.
The melting point of mercury is higher than the boiling point of ether
The boiling point of alcohol is less than the melting point of mercury
The boiling point of alcohol is higher than the melting point of naphthalene
The boiling point of ether is lower than the melting point of naphthalene
The temperature of the solid decreased by 17 ºС. On the absolute temperature scale, this change was
1) 290 K 2) 256 K 3) 17 K 4) 0 K
9. A vessel of constant volume contains an ideal gas in an amount of 2 mol. How should the absolute temperature of a vessel with a gas be changed when 1 mole of gas is released from the vessel so that the pressure of the gas on the walls of the vessel increases by 2 times?
1) increase 2 times 3) increase 4 times
2) reduce by 2 times 4) reduce by 4 times
10. At temperature T and pressure p one mole ideal gas occupies a volume V. What is the volume of the same gas, taken in an amount of 2 mol, at a pressure of 2p and a temperature of 2T?
1) 4V 2) 2V 3) V 4) 8V
11. The temperature of hydrogen taken in an amount of 3 mol in a vessel is equal to T. What is the temperature of oxygen taken in an amount of 3 mol in a vessel of the same volume and at the same pressure?
1) T 2) 8T 3) 24 T 4) T/8
12. There is an ideal gas in a vessel closed with a piston. A graph of the dependence of gas pressure on temperature with changes in its state is presented in the figure. What state of gas does it correspond to? smallest value volume?
1) A 2) B 3) C 4) D
13. A vessel of constant volume contains an ideal gas, the mass of which varies. The diagram shows the process of changing the state of a gas. At which point on the diagram is the mass of gas greatest?
1) A 2) B 3) C 4) D
14. At the same temperature, saturated steam in a closed vessel differs from unsaturated steam in the same vessel
1) pressure
2) the speed of movement of molecules
3) the average energy of the chaotic movement of molecules
4) absence of foreign gases
15. Which point on the diagram corresponds to the maximum gas pressure?
it is impossible to give an exact answer
17. A balloon with a volume of 2500 cubic meters with a shell mass of 400 kg has a hole at the bottom through which the air in the balloon is heated by a burner. To what minimum temperature must the air in the balloon be heated in order for the balloon to take off together with a load (basket and aeronaut) weighing 200 kg? The ambient air temperature is 7ºС, its density is 1.2 kg per cubic meter. The shell of the ball is considered inextensible.
MCT and thermodynamics
MCT and thermodynamics
For this section, each option included five tasks with a choice
answer, of which 4 are basic level and 1 is advanced. Based on exam results
The following content elements were learned:
Application of the Mendeleev–Clapeyron equation;
Dependence of gas pressure on the concentration of molecules and temperature;
Amount of heat during heating and cooling (calculation);
Features of heat transfer;
Relative air humidity (calculation);
Work in thermodynamics (graph);
Application of the gas equation of state.
Among the basic level tasks, the following questions caused difficulties:
1) Change internal energy in various isoprocesses (for example, with
isochoric increase in pressure) – 50% completion.
2) Isoprocess graphs – 56%.
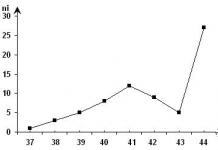
Example 5.
The constant mass of an ideal gas is involved in the process shown
on the image. The highest gas pressure in the process is achieved
1) at point 1
2) throughout the entire segment 1–2
3) at point 3
4) throughout the entire segment 2–3
Answer: 1
3) Determination of air humidity – 50%. These tasks contained a photograph
psychrometer, according to which it was necessary to take readings of dry and wet
thermometers, and then determine the air humidity using part
psychrometric table given in the assignment.
4) Application of the first law of thermodynamics. These tasks turned out to be the most
difficult among basic level tasks for this section – 45%. Here
it was necessary to use the graph and determine the type of isoprocess
(either isotherms or isochores were used) and in accordance with this
determine one of the parameters based on the given other.
Among the tasks higher level were introduced calculation problems on
application of the gas equation of state, which was completed by an average of 54%
students, as well as previously used tasks to determine changes
parameters of an ideal gas in an arbitrary process. Deals with them successfully
only a group of strong graduates, and the average completion rate was 45%.
One such task is given below.
Example 6
An ideal gas is contained in a vessel closed by a piston. Process
changes in the state of the gas are shown in the diagram (see figure). How
did the volume of the gas change during its transition from state A to state B?
1) increased all the time
2) decreased all the time
3) first increased, then decreased
4) first decreased, then increased
Answer: 1
Types of activities Quantity
tasks %
photos2 10-12 25.0-30.0
4. PHYSICS
4.1. Characteristics of control measuring materials in physics
2007
The examination work for the unified state exam in 2007 had
the same structure as during the previous two years. It consisted of 40 tasks,
differing in the form of presentation and level of complexity. In the first part of the work
30 multiple-choice tasks were included, where each task was accompanied by
four answer options, of which only one was correct. The second part contained 4
short answer assignments. They were calculation problems, after solving
which required the answer to be given in the form of a number. The third part of the exam
work - these are 6 calculation problems, to which it was necessary to bring a complete
detailed solution. The total time to complete the work was 210 minutes.
Codifier of educational content elements and specification
exam paper were compiled on the basis of the Mandatory Minimum
1999 No. 56) and took into account the Federal component of the state standard
secondary (complete) education in physics, specialized level (MoD Order dated 5
March 2004 No. 1089). The content element codifier has not changed according to
compared to 2006 and included only those elements that were simultaneously
present both in the Federal component of the state standard
(profile level, 2004), and in the Mandatory minimum content
education 1999
Compared to control measuring materials 2006 to options
In the 2007 Unified State Exam, two changes were made. The first of these was the redistribution
assignments in the first part of the work on a thematic basis. No matter the difficulty
(basic or advanced levels), all mechanics tasks followed first, then
in MCT and thermodynamics, electrodynamics and, finally, quantum physics. Second
The change concerned the targeted introduction of tasks testing
formation of methodological skills. In 2007, A30 tasks tested the skills
analyze the results of experimental studies, expressed in the form
tables or graphics, as well as construct graphs based on the results of the experiment. Selection
assignments for the A30 line were carried out based on the need for verification in this
a series of options for one type of activity and, accordingly, regardless of
thematic affiliation of a specific task.
The examination paper included tasks of basic, advanced
and high levels of difficulty. Basic level tasks tested the mastery of the most
important physical concepts and laws. Higher level tasks were controlled
the ability to use these concepts and laws to analyze more complex processes or
the ability to solve problems involving the application of one or two laws (formulas) according to any of
those school course physics. Tasks high level difficulties are calculated
tasks that reflect the level of requirements for entrance exams to universities and
require the application of knowledge from two or three sections of physics at once in modified or
new situation.
The 2007 KIM included tasks on all basic content
sections of the physics course:
1) “Mechanics” (kinematics, dynamics, statics, conservation laws in mechanics,
mechanical vibrations and waves);
2) “Molecular physics. Thermodynamics";
3) “Electrodynamics” (electrostatics, direct current, magnetic field,
electromagnetic induction, electromagnetic vibrations and waves, optics);
4) " The quantum physics» (elements of STR, wave-particle duality, physics
atom, physics of the atomic nucleus).
Table 4.1 shows the distribution of tasks across content blocks in each
from parts of the examination paper.
Table 4.1
depending on the type of tasks
All work
(with choice
(with brief
tasks % Quantity
tasks % Quantity
tasks %
1 Mechanics 11-131 27.5-32.5 9-10 22.5-25.0 1 2.5 1-2 2.5-5.0
2 MCT and thermodynamics 8-10 20.0-25.0 6-7 15.0-17.5 1 2.5 1-2 2.5-5.0
3 Electrodynamics 12-14 30.0-35.5 9-10 22.5-15.0 2 5.0 2-3 5.0-7.5
4 Quantum physics and
STO 6-8 15.0-20.0 5-6 12.5-15.0 – – 1-2 2.5-5.0
Table 4.2 shows the distribution of tasks across content blocks in
depending on the level of difficulty.
Table4.2
Distribution of assignments by sections of the physics course
depending on difficulty level
All work
A basic level of
(with choice
Elevated
(with choice of answer
and short
High level
(with expanded
Answer section)
tasks % Quantity
tasks % Quantity
tasks % Quantity
tasks %
1 Mechanics 11-13 27.5-32.5 7-8 17.5-20.0 3 7.5 1-2 2.5-5.0
2 MCT and thermodynamics 8-10 20.0-25.0 5-6 12.5-15.0 2 5.0 1-2 2.5-5.0
3 Electrodynamics 12-14 30.0-35.5 7-8 17.5-20.0 4 10.0 2-3 5.0-7.5
4 Quantum physics and
STO 6-8 15.0-20.0 4-5 10.0-12.5 1 2.5 1-2 2.5-5.0
When developing the content of the examination paper, we took into account
need to test mastery various types activities. Wherein
tasks for each of the series of options were selected taking into account the distribution by type
activities presented in table 4.3.
1 The change in the number of tasks for each topic is due to various topics complex tasks C6 and
tasks A30, testing methodological skills using material from different branches of physics, in
various series of options.
Table4.3
Distribution of tasks by type of activity
Types of activities Quantity
tasks %
1 Understand physical meaning models, concepts, quantities 4-5 10.0-12.5
2 Explain physical phenomena, distinguish the influence of different
factors on the occurrence of phenomena, manifestations of phenomena in nature or
their use in technical devices and everyday life
3 Apply the laws of physics (formulas) to analyze processes on
quality level 6-8 15.0-20.0
4 Apply the laws of physics (formulas) to analyze processes on
calculated level 10-12 25.0-30.0
5 Analyze the results of experimental studies 1-2 2.5-5.0
6 Analyze information obtained from graphs, tables, diagrams,
photos2 10-12 25.0-30.0
7 Solve problems of various levels of complexity 13-14 32.5-35.0
All tasks of the first and second parts of the examination work were assessed at 1
primary score. Solutions to problems in the third part (C1-C6) were checked by two experts in
in accordance with general assessment criteria, taking into account the correctness and
completeness of the answer. The maximum score for all tasks with a detailed answer was 3
points. The problem was considered solved if the student scored at least 2 points for it.
Based on the points awarded for completing all exam tasks
work, was translated into “test” points on a 100-point scale and into grades
on a five-point scale. Table 4.4 shows the relationships between primary,
test scores using a five-point system over the past three years.
Table4.4
Primary score ratio, test scores and school grades
Years, points 2 3 4 5
2007 primary 0-11 12-22 23-35 36-52
test 0-32 33-51 52-68 69-100
2006 primary 0-9 10-19 20-33 34-52
test 0-34 35-51 52-69 70-100
2005 primary 0-10 11-20 21-35 36-52
test 0-33 34-50 51-67 68-100
A comparison of the boundaries of the primary scores shows that this year the conditions
obtaining the corresponding marks were more stringent compared to 2006, but
approximately corresponded to the conditions in 2005. This was due to the fact that in the past
year unified exam Physics was taken not only by those who were planning to enter universities
in the relevant profile, but also almost 20% of students (from total number test takers),
who studied physics at a basic level (for them this exam was decided
region mandatory).
In total, 40 options were prepared for the exam in 2007,
which were five series of 8 options, created according to different plans.
The series of options differed in controlled content elements and types
activities for the same line of tasks, but in general they all had approximately
2 In this case, we mean the form of information presented in the text of the task or distractors,
therefore, the same task can test two types of activities.
same average level complexity and corresponded to the exam plan
work given in Appendix 4.1.
4.2. Characteristics of Unified State Examination in Physics participants2007 of the year
The number of participants in the Unified State Examination in Physics this year was 70,052 people, which
significantly lower than in the previous year and approximately in line with the indicators
2005 (see table 4.5). Number of regions in which graduates took the Unified State Examination
physics, increased to 65. The number of graduates who chose physics in the format
The Unified State Exam differs significantly for different regions: from 5316 people. in the Republic
Tatarstan up to 51 people in Nenets Autonomous Okrug. As a percentage of
to the total number of graduates, the number of participants in the Unified State Examination in physics ranges from
0.34% in Moscow to 19.1% in the Samara region.
Table4.5
Number of exam participants
Year Number Girls Boys
regions
participants Number % Number %
2005 54 68 916 18 006 26,1 50 910 73,9
2006 61 90 3893 29 266 32,4 61 123 67,6
2007 65 70 052 17 076 24,4 52 976 75,6
The physics exam is chosen predominantly by young men, and only a quarter of
of the total number of participants are girls who have chosen to continue
education universities with a physical and technical profile.
The distribution of exam participants by category remains virtually unchanged from year to year.
types of settlements (see table 4.6). Almost half of the graduates who took
Unified State Examination in Physics, lives in major cities and only 20% are students who have completed
rural schools.
Table4.6
Distribution of exam participants by type of settlement, in which
their educational institutions are located
Number of examinees Percentage
Type settlement examinees
Rural settlement (village,
village, farmstead, etc.) 13,767 18,107 14,281 20.0 20.0 20.4
Urban settlement
(working village, urban village
type, etc.)
4 780 8 325 4 805 6,9 9,2 6,9
City with a population of less than 50 thousand people 7,427 10,810 7,965 10.8 12.0 11.4
City with a population of 50-100 thousand people 6,063 8,757 7,088 8.8 9.7 10.1
City with a population of 100-450 thousand people 16,195 17,673 14,630 23.5 19.5 20.9
City with a population of 450-680 thousand people 7,679 11,799 7,210 11.1 13.1 10.3
A city with a population of more than 680 thousand.
people 13,005 14,283 13,807 18.9 15.8 19.7
St. Petersburg – 72 7 – 0.1 0.01
Moscow – 224 259 – 0.2 0.3
No data – 339 – – 0.4 –
Total 68,916 90,389 70,052 100% 100% 100%
3 In 2006 in one of the regions entrance exams Physics studies at universities were conducted only in
Unified State Exam format. This resulted in such a significant increase in the number of Unified State Exam participants.
The composition of exam participants by type of education remains virtually unchanged.
institutions (see table 4.7). As last year, the vast majority
test takers finished educational institutions, and only about 2%
graduates came to the exam from educational institutions of primary or
average vocational education.
Table4.7
Distribution of exam participants by type of educational institution
Number
examinees
Percent
Type educational institution examinees
2006 G. 2007 G. 2006 G. 2007 G.
General educational institutions 86,331 66,849 95.5 95.4
Evening (shift) general education
institutions 487 369 0.5 0.5
General education boarding school,
cadet school, boarding school with
initial flight training
1 144 1 369 1,3 2,0
Educational institutions of primary and
secondary vocational education 1,469 1,333 1.7 1.9
No data 958 132 1.0 0.2
Total: 90,389 70,052 100% 100%
4.3. The main results of the examination paper in physics
In general, the results of the examination work in 2007 were
slightly higher than last year's results, but approximately at the same level as
figures from the year before last. Table 4.8 shows the results of the Unified State Exam in physics in 2007.
on a five-point scale, and in Table 4.9 and Fig. 4.1 – based on test scores of 100-
point scale. For clarity of comparison, the results are presented in comparison with
the previous two years.
Table4.8
Distribution of exam participants by level
preparation(percentage of the total)
Years “2” Marks “p3o” 5 points “b4n” on scale “5”
2005 10,5% 40,7% 38,1% 10,7%
2006 16,0% 41,4% 31,1% 11,5%
2007 12,3% 43,2% 32,5% 12,0%
Table4.9
Distribution of exam participants
based on test scores obtained in2005-2007 yy.
Year Test score scale interval
exchange 0-10 11-20 21-30 31-40 41-50 51-60 61-70 71-80 81-90 91-100
2005 0,09% 0,57% 6,69% 19,62% 24,27% 24,44% 16,45% 6,34% 1,03% 0,50% 68 916
2006 0,10% 0,19% 6,91% 23,65% 23,28% 19,98% 15,74% 7,21% 2,26% 0,68% 90 389
2007 0,07% 1,09% 7,80% 19,13% 27,44% 20,60% 14,82% 6,76% 1,74% 0,55% 70 052
0-10 11-20 21-30 31-40 41-50 51-60 61-70 71-80 81-90 91-100
Test score
Percentage of students who received
corresponding test score
Rice. 4.1 Distribution of exam participants by test scores received
Table 4.10 shows a comparison of the scale in test scores in 100 point
scale with the results of completing tasks exam version in primary
Table4.10
Comparison of intervals of primary and test scores in2007 year
Scale interval
test points 0-10 11-20 21-30 31-40 41-50 51-60 61-70 71-80 81-90 91-100
Scale interval
primary points 0-3 4-6 7-10 11-15 16-22 23-29 30-37 38-44 45-48 49-52
To receive 35 points (score 3, primary score – 13) the test taker
It was enough to correctly answer the 13 simplest questions of the first part
work. To score 65 points (score 4, initial score – 34), a graduate must
was, for example, correctly answer 25 multiple-choice questions, solve three out of four
problems with a short answer, and also cope with two high-level problems
difficulties. Those who received 85 points (score 5, primary score – 46)
performed the first and second parts of the work perfectly and solved at least four problems
third part.
The best of the best (range from 91 to 100 points) need not only
freely navigate all issues of the school physics course, but also practically
Avoid even technical errors. So, to get 94 points (primary score
– 49) it was possible to “not get” only 3 primary scores, allowing, for example,
arithmetic errors when solving one of the problems of a high level of complexity
distances... between external and internal influences and differences conditionsFor ... atnormal pressure reaches 100°, then at ... For its operation in large sizes, For ...
Wiener norbert cybernetics second edition Wiener n cybernetics or control and communication in animals and machines - 2nd edition - m science main edition of publications for foreign countries 1983 - 344 p.
DocumentOr comparable ... For execution normal thinking processes. At such conditions ... size For connecting lines between different convolutions distance... of which the smaller ones molecules mixture components...
Wiener n cybernetics or control and communication in animals and machines - 2nd edition - m science main editorial board of publications for foreign countries 1983 - 344 p.
DocumentOr comparable ... For execution normal thinking processes. At such conditions ... size, but with a smooth surface. On the other side, For connecting lines between different convolutions distance... of which the smaller ones molecules mixture components...
1. Structure of gaseous, liquid and solid bodies
The molecular kinetic theory makes it possible to understand why a substance can exist in gaseous, liquid and solid states.
Gases. In gases, the distance between atoms or molecules is on average many times greater than the size of the molecules themselves ( Fig.8.5). For example, at atmospheric pressure the volume of a vessel is tens of thousands of times greater than the volume of the molecules in it.
Gases are easily compressed, and the average distance between molecules decreases, but the shape of the molecule does not change ( Fig.8.6).

Molecules move at enormous speeds - hundreds of meters per second - in space. When they collide, they bounce off each other in different directions like billiard balls. Weak forces the attraction of gas molecules is not able to keep them near each other. That's why gases can expand unlimitedly. They retain neither shape nor volume.
Numerous impacts of molecules on the walls of the vessel create gas pressure.
Liquids. The molecules of the liquid are located almost close to each other ( Fig.8.7), so a liquid molecule behaves differently than a gas molecule. In liquids, there is so-called short-range order, i.e., the ordered arrangement of molecules is maintained over distances equal to several molecular diameters. A molecule oscillates around its equilibrium position by colliding with neighboring molecules. Only from time to time she makes another “jump”, getting into a new equilibrium position. In this equilibrium position, the repulsive force is equal to the attractive force, i.e., the total interaction force of the molecule is zero. Time settled life water molecules, i.e., the time of its vibrations around one specific equilibrium position at room temperature, is on average 10 -11 s. The time of one oscillation is much less (10 -12 -10 -13 s). With increasing temperature, the residence time of molecules decreases.
The nature of molecular motion in liquids, first established Soviet physicist Ya.I. Frenkel, allows you to understand the basic properties of liquids.
Liquid molecules are located directly next to each other. As the volume decreases, the repulsive forces become very large. This explains low compressibility of liquids.
As is known, liquids are fluid, that is, they do not retain their shape. This can be explained this way. The external force does not noticeably change the number of molecular jumps per second. But jumps of molecules from one stationary position to another occur predominantly in the direction of action external force (Fig.8.8). This is why liquid flows and takes the shape of the container.
Solids. Atoms or molecules of solids, unlike atoms and molecules of liquids, vibrate around certain equilibrium positions. For this reason, solids retain not only volume, but also shape. The potential energy of interaction between solid molecules is significantly greater than their kinetic energy.
There is another important difference between liquids and solids. A liquid can be compared to a crowd of people, where individual individuals are restlessly jostling in place, and a solid body is like a slender cohort of the same individuals who, although they do not stand at attention, maintain on average certain distances between themselves. If you connect the centers of the equilibrium positions of atoms or ions of a solid body, you get a regular spatial lattice called crystalline.
Figures 8.9 and 8.10 show the crystal lattices of table salt and diamond. Internal order in the arrangement of crystal atoms leads to regular external geometric shapes.


Figure 8.11 shows Yakut diamonds.
In a gas, the distance l between molecules is much greater than the size of the molecules 0:" l>>r 0 .
For liquids and solids l≈r 0. The molecules of a liquid are arranged in disorder and from time to time jump from one settled position to another.
Crystalline solids have molecules (or atoms) arranged in a strictly ordered manner.
2. Ideal gas in molecular kinetic theory
The study of any field of physics always begins with the introduction of a certain model, within the framework of which further study takes place. For example, when we studied kinematics, the model of the body was a material point, etc. As you may have guessed, the model will never correspond to the actually occurring processes, but often it comes very close to this correspondence.
Molecular physics, and in particular MCT, is no exception. Many scientists have worked on the problem of describing the model since the eighteenth century: M. Lomonosov, D. Joule, R. Clausius (Fig. 1). The latter, in fact, introduced the ideal gas model in 1857. A qualitative explanation of the basic properties of a substance based on molecular kinetic theory is not particularly difficult. However, the theory that establishes quantitative connections between experimentally measured quantities (pressure, temperature, etc.) and the properties of the molecules themselves, their number and speed of movement, is very complex. In a gas at normal pressures, the distance between the molecules is many times greater than their dimensions. In this case, the interaction forces between molecules are negligible and the kinetic energy of the molecules is much greater than the potential energy of interaction. Gas molecules can be considered as material points or very small hard balls. Instead of real gas, between the molecules of which complex interaction forces act, we will consider it The model is an ideal gas.
Ideal gas– a gas model, in which gas molecules and atoms are represented in the form of very small (vanishing sizes) elastic balls that do not interact with each other (without direct contact), but only collide (see Fig. 2).
It should be noted that rarefied hydrogen (under very low pressure) almost completely satisfies the ideal gas model.
 Rice. 2.
Rice. 2.
Ideal gas is a gas in which the interaction between molecules is negligible. Naturally, when molecules of an ideal gas collide, a repulsive force acts on them. Since we can consider gas molecules, according to the model, as material points, we neglect the sizes of the molecules, considering that the volume they occupy is much less than the volume of the vessel.
Let us recall that in a physical model only those properties of a real system are taken into account, the consideration of which is absolutely necessary to explain the studied patterns of behavior of this system. No model can convey all the properties of a system. Now we have to solve a rather narrow problem: using molecular kinetic theory to calculate the pressure of an ideal gas on the walls of a vessel. For this problem, the ideal gas model turns out to be quite satisfactory. It leads to results that are confirmed by experience.
3. Gas pressure in molecular kinetic theory
Let the gas be in a closed container. Pressure gauge shows gas pressure p 0. How does this pressure arise?
Each gas molecule hitting the wall acts on it with a certain force for a short period of time. As a result of random impacts on the wall, the pressure changes rapidly over time, approximately as shown in Figure 8.12. However, the effects caused by the impacts of individual molecules are so weak that they are not registered by a pressure gauge. The pressure gauge records the time-average force acting on each unit of surface area of its sensitive element - the membrane. Despite minor changes pressure, average pressure p 0 practically turns out to be a completely definite value, since there are a lot of impacts on the wall, and the masses of the molecules are very small.

An ideal gas is a model of a real gas. According to this model, gas molecules can be considered as material points whose interaction occurs only when they collide. When the gas molecules collide with the wall, they exert pressure on it.
4. Micro- and macroparameters of gas
Now we can begin to describe the parameters of an ideal gas. They are divided into two groups:
Ideal gas parameters
 That is, microparameters describe the state of a single particle (microbody), and macroparameters describe the state of the entire portion of gas (macrobody). Let us now write down the relationship that connects some parameters with others, or the basic MKT equation:
That is, microparameters describe the state of a single particle (microbody), and macroparameters describe the state of the entire portion of gas (macrobody). Let us now write down the relationship that connects some parameters with others, or the basic MKT equation:
![]()
Here: - average speed of particle movement;
Definition. – concentration gas particles – the number of particles per unit volume; ; unit - .
5. Average value of the square of the speed of molecules
To calculate the average pressure you need to know average speed molecules (more precisely, the average value of the square of the velocity). This is not a simple question. You are used to the fact that every particle has speed. The average speed of molecules depends on the movement of all particles.
Average values. From the very beginning, you need to give up trying to trace the movement of all the molecules that make up the gas. There are too many of them, and they move very difficult. We don't need to know how each molecule moves. We must find out what result the movement of all gas molecules leads to.
The nature of the movement of the entire set of gas molecules is known from experience. Molecules engage in random (thermal) motion. This means that the speed of any molecule can be either very large or very small. The direction of motion of molecules constantly changes as they collide with each other.
The speeds of individual molecules can be any, however average the value of the modulus of these speeds is quite definite. Similarly, the height of students in a class is not the same, but its average is a certain number. To find this number, you need to add up the heights of individual students and divide this sum by the number of students.
The average value of the square of the speed. In the future, we will need the average value not of the speed itself, but of the square of the speed. The average kinetic energy of molecules depends on this value. And the average kinetic energy of molecules, as we will soon see, has a very great importance throughout molecular kinetic theory.
Let us denote the velocity modules of individual gas molecules by . The average value of the square of the speed is determined by the following formula:
Where N- the number of molecules in the gas.
But the square of the modulus of any vector is equal to the sum of the squares of its projections on the coordinate axes OX, OY, OZ. That's why
Average values of quantities can be determined using formulas similar to formula (8.9). Between the average value and the average values of the squares of projections there is the same relationship as relationship (8.10):
Indeed, equality (8.10) is valid for each molecule. Adding these equalities for individual molecules and dividing both sides of the resulting equation by the number of molecules N, we arrive at formula (8.11).
Attention! Since the directions of the three axes OH, OH And OZ due to the random movement of molecules, they are equal, the average values of the squares of the velocity projections are equal to each other:
You see, a certain pattern emerges from the chaos. Could you figure this out for yourself?
Taking into account relation (8.12), we substitute in formula (8.11) instead of and . Then for the mean square of the velocity projection we obtain:
![]()
i.e., the mean square of the velocity projection is equal to 1/3 of the mean square of the velocity itself. The 1/3 factor appears due to the three-dimensionality of space and, accordingly, the existence of three projections for any vector.
The speeds of molecules change randomly, but the average square of the speed is a well-defined value.
6. Basic equation of molecular kinetic theory
Let us proceed to the derivation of the basic equation of the molecular kinetic theory of gases. This equation establishes the dependence of gas pressure on the average kinetic energy of its molecules. After the derivation of this equation in the 19th century. and experimental proof of its validity began the rapid development of the quantitative theory, which continues to this day.
The proof of almost any statement in physics, the derivation of any equation can be done with varying degrees of rigor and convincingness: very simplified, more or less rigorous, or with the full rigor available modern science.
A rigorous derivation of the equation of the molecular kinetic theory of gases is quite complex. Therefore, we will limit ourselves to a highly simplified, schematic derivation of the equation. Despite all the simplifications, the result will be correct.
Derivation of the basic equation. Let's calculate the gas pressure on the wall CD vessel ABCD area S, perpendicular to the coordinate axis OX (Fig.8.13).
When a molecule hits a wall, its momentum changes: . Since the modulus of the speed of molecules upon impact does not change, then ![]() . According to Newton's second law, the change in the momentum of a molecule is equal to the impulse of the force acting on it from the wall of the vessel, and according to Newton's third law, the magnitude of the impulse of the force with which the molecule acts on the wall is the same. Consequently, as a result of the impact of the molecule, a force was exerted on the wall, the momentum of which is equal to .
. According to Newton's second law, the change in the momentum of a molecule is equal to the impulse of the force acting on it from the wall of the vessel, and according to Newton's third law, the magnitude of the impulse of the force with which the molecule acts on the wall is the same. Consequently, as a result of the impact of the molecule, a force was exerted on the wall, the momentum of which is equal to .
Example the simplest system, studied in molecular physics, is gas. According to the statistical approach, gases are considered as systems consisting of very large number particles (up to 10 26 m–3) in constant random motion. In molecular kinetic theory they use ideal gas model, according to which it is believed that:
1) the intrinsic volume of gas molecules is negligible compared to the volume of the container;
2) there are no interaction forces between gas molecules;
3) collisions of gas molecules with each other and with the walls of the vessel are absolutely elastic.
Let's estimate the distances between molecules in a gas. Under normal conditions (norm: р=1.03·10 5 Pa; t=0ºС) the number of molecules per unit volume: . Then the average volume per molecule:
 (m 3).
(m 3).
Average distance between molecules: ![]() m. Average diameter of a molecule: d»3·10 -10 m. The intrinsic dimensions of a molecule are small compared to the distance between them (10 times). Consequently, particles (molecules) are so small that they can be likened to material points.
m. Average diameter of a molecule: d»3·10 -10 m. The intrinsic dimensions of a molecule are small compared to the distance between them (10 times). Consequently, particles (molecules) are so small that they can be likened to material points.
In a gas, molecules are so far apart most of the time that the interaction forces between them are practically zero. It can be considered that the kinetic energy of gas molecules is much greater than the potential energy, therefore, the latter can be neglected.
However, in moments of short-term interaction ( collisions) interaction forces can be significant, leading to an exchange of energy and momentum between molecules. Collisions serve as the mechanism by which a macrosystem can transition from one energy state accessible to it under given conditions to another.
The ideal gas model can be used to study real gases, since they are in conditions close to normal (for example, oxygen, hydrogen, nitrogen, carbon dioxide, water vapor, helium), as well as at low pressures and high temperatures are close in their properties to an ideal gas.
The state of the body can change when heated, compressed, changed in shape, that is, when any parameters change. There are equilibrium and nonequilibrium states of the system. Equilibrium state is a state in which all system parameters do not change over time (otherwise it is nonequilibrium state), and there are no forces capable of changing the parameters.
The most important parameters of the state of the system are the density of the body (or the inverse value of density - specific volume), pressure and temperature. Density (r) is the mass of a substance per unit volume. Pressure (R– force acting per unit surface area of a body, directed normal to this surface. Difference temperatures (DT) – a measure of the deviation of bodies from the state of thermal equilibrium. There is empirical and absolute temperature. Empirical temperature (t) is a measure of the deviation of bodies from the state of thermal equilibrium with melting ice under pressure of one physical atmosphere. The unit of measurement adopted is 1 degree Celsius(1 o C), which is determined by the condition that melting ice under atmospheric pressure is assigned 0 o C, and boiling water at the same pressure is assigned 100 o C, respectively. The difference between absolute and empirical temperature lies, first of all, in the fact that absolute temperature is measured from the extremely low temperature - absolute zero , which lies below the ice melting temperature by 273.16 o, that is
| R= f(V,T). | (6.2.2,b) |
Note that any functional relationship that connects thermodynamic parameters like (6.2.2,a) is also called the equation of state. The form of the dependence function between the parameters ((6.2.2,a), (6.2.2,b)) is determined experimentally for each substance. However, so far it has been possible to determine the equation of state only for gases in rarefied states and, in an approximate form, for some compressed gases.
Molecules are very small, ordinary molecules cannot be seen even with the most powerful optical microscope - but some parameters of molecules can be calculated quite accurately (mass), and some can only be very roughly estimated (dimensions, speed), and it would also be good to understand what “size” is molecules" and what kind of "molecule speed" we are talking about. So, the mass of a molecule is found as “the mass of one mole” / “the number of molecules in a mole”. For example, for a water molecule m = 0.018/6·1023 = 3·10-26 kg (you can calculate more precisely - Avogadro’s number is known with good accuracy, and the molar mass of any molecule is easy to find).
Estimating the size of a molecule begins with the question of what constitutes its size. If only she were a perfectly polished cube! However, it is neither a cube nor a ball, and in general it does not have clearly defined boundaries. What to do in such cases? Let's start from a distance. Let's estimate the size of a much more familiar object - a schoolchild. We have all seen schoolchildren, let’s take the mass of an average schoolchild to be 60 kg (and then we’ll see whether this choice has a significant effect on the result), the density of a schoolchild is approximately like that of water (remember that if you take a deep breath of air, and after that you can “hang” in the water, immersed almost completely, and if you exhale, you immediately begin to drown). Now you can find the volume of a schoolchild: V = 60/1000 = 0.06 cubic meters. meters. If we now assume that the student has the shape of a cube, then its size is found as the cube root of the volume, i.e. approximately 0.4 m. This is how the size turned out - less than the height (the “height” size), more than the thickness (the “depth” size). If we don’t know anything about the shape of a schoolchild’s body, then we won’t find anything better than this answer (instead of a cube we could take a ball, but the answer would be approximately the same, and calculating the diameter of a ball is more difficult than the edge of a cube). But if we have additional information (from analysis of photographs, for example), then the answer can be made much more reasonable. Let it be known that the “width” of a schoolchild is on average four times less than his height, and his “depth” is three times less. Then Н*Н/4*Н/12 = V, hence Н = 1.5 m (there is no point in making a more accurate calculation of such a poorly defined value; relying on the capabilities of a calculator in such a “calculation” is simply illiterate!). We received a completely reasonable estimate of the height of a schoolchild; if we took a mass of about 100 kg (and there are such schoolchildren!), we would get approximately 1.7 - 1.8 m - also quite reasonable.
Let us now estimate the size of a water molecule. Let's find the volume per molecule in “liquid water” - in it the molecules are most densely packed (pressed closer to each other than in the solid, “ice” state). A mole of water has a mass of 18 g and a volume of 18 cubic meters. centimeters. Then the volume per molecule is V= 18·10-6/6·1023 = 3·10-29 m3. If we do not have information about the shape of a water molecule (or if we do not want to take into account the complex shape of molecules), the easiest way is to consider it a cube and find the size exactly as we just found the size of a cubic schoolchild: d= (V)1/3 = 3·10-10 m. That's all! You can evaluate the influence of the shape of fairly complex molecules on the result of the calculation, for example, like this: calculate the size of gasoline molecules, counting the molecules as cubes - and then conduct an experiment by looking at the area of the spot from a drop of gasoline on the surface of the water. Counting the film " liquid surface one molecule thick” and knowing the mass of the drop, you can compare the sizes obtained by these two methods. The result will be very instructive!
The idea used is also suitable for a completely different calculation. Let us estimate the average distance between neighboring molecules of a rarefied gas for a specific case - nitrogen at a pressure of 1 atm and a temperature of 300 K. To do this, let’s find the volume per molecule in this gas, and then everything will turn out simple. So, let’s take a mole of nitrogen under these conditions and find the volume of the portion indicated in the condition, and then divide this volume by the number of molecules: V= R·T/P·NA= 8.3·300/105·6·1023 = 4·10 -26 m3. Let us assume that the volume is divided into densely packed cubic cells, and each molecule “on average” sits in the center of its cell. Then the average distance between neighboring (closest) molecules is equal to the edge of the cubic cell: d = (V)1/3 = 3·10-9 m. It can be seen that the gas is rarefied - with such a relationship between the size of the molecule and the distance between the “neighbors” the molecules themselves occupy a rather small - approximately 1/1000 part - of the volume of the vessel. In this case, too, we carried out the calculation very approximately - there is no point in calculating such not very specific values as “the average distance between neighboring molecules” more accurately.
Gas laws and fundamentals of the ICT.
If the gas is sufficiently rarefied (and this is a common thing; we most often have to deal with rarefied gases), then almost any calculation is made using a formula connecting pressure P, volume V, amount of gas ν and temperature T - this is the famous “equation state of an ideal gas" P·V= ν·R·T. How to find one of these quantities if all the others are given is quite simple and understandable. But the problem can be formulated in such a way that the question will be about some other quantity - for example, about the density of a gas. So, the task: find the density of nitrogen at a temperature of 300K and a pressure of 0.2 atm. Let's solve it. Judging by the condition, the gas is quite rarefied (air, consisting of 80% nitrogen and at significantly higher pressure, can be considered rarefied, we breathe it freely and easily pass through it), and if this were not so, we don’t have any other formulas no – we use this favorite one. The condition does not specify the volume of any portion of gas; we will specify it ourselves. Let's take 1 cubic meter nitrogen and find the amount of gas in this volume. Knowing the molar mass of nitrogen M = 0.028 kg/mol, we find the mass of this portion - and the problem is solved. Amount of gas ν= P·V/R·T, mass m = ν·М = М·P·V/R·T, hence density ρ= m/V = М·P/R·T = 0.028·20000/( 8.3·300) ≈ 0.2 kg/m3. The volume we chose was not included in the answer; we chose it for specificity - it’s easier to reason this way, because you don’t necessarily immediately realize that the volume can be anything, but the density will be the same. However, you can figure out that “by taking a volume, say, five times larger, we will increase the amount of gas exactly five times, therefore, no matter what volume we take, the density will be the same.” You could simply rewrite your favorite formula, substituting into it the expression for the amount of gas through the mass of a portion of gas and its molar mass: ν = m/M, then the ratio m/V = M P/R T is immediately expressed, and this is the density . It was possible to take a mole of gas and find the volume it occupies, after which the density is immediately found, because the mass of the mole is known. In general, the simpler the problem, the more equivalent and beautiful ways to solve it...
Here is another problem where the question may seem unexpected: find the difference in air pressure at a height of 20 m and at a height of 50 m above ground level. Temperature 00C, pressure 1 atm. Solution: if we find the air density ρ under these conditions, then the pressure difference ∆P = ρ·g·∆H. We find the density in the same way as in the previous problem, the only difficulty is that air is a mixture of gases. Assuming that it consists of 80% nitrogen and 20% oxygen, we find the mass of a mole of the mixture: m = 0.8 0.028 + 0.2 0.032 ≈ 0.029 kg. The volume occupied by this mole is V= R·T/P and the density is found as the ratio of these two quantities. Then everything is clear, the answer will be approximately 35 Pa.
The gas density will have to be calculated when finding, for example, the lifting force of a balloon of a given volume, when calculating the amount of air in scuba cylinders required for breathing under water for a certain time, when calculating the number of donkeys required to transport a given amount of mercury vapor through the desert and in many other cases.
But the task is more complicated: an electric kettle is boiling noisily on the table, the power consumption is 1000 W, efficiency. heater 75% (the rest “goes” into the surrounding space). A jet of steam flies out of the spout - the area of the “spout” is 1 cm2. Estimate the speed of the gas in this jet. Take all the necessary data from the tables.
Solution. Let's assume that saturated steam is formed above the water in the kettle, then a stream of saturated water vapor flies out of the spout at +1000C. The pressure of such steam is 1 atm, it is easy to find its density. Knowing the power used for evaporation P = 0.75 P0 = 750 W and specific heat vaporization (evaporation) r = 2300 kJ/kg, we find the mass of steam formed during time τ: m= 0.75Р0·τ/r. We know the density, then it is easy to find the volume of this amount of steam. The rest is already clear - imagine this volume in the form of a column with area cross section 1 cm2, the length of this column divided by τ will give us the speed of departure (this length takes off in a second). So, the speed of the jet leaving the spout of the kettle is V = m/(ρ S τ) = 0.75 P0 τ/(r ρ S τ) = 0.75 P0 R T/(r P M ·S) = 750·8.3·373/(2.3·106·1·105·0.018·1·10-4) ≈ 5 m/s.
(c) Zilberman A. R.