The story about gaseous compounds of phosphorus, and above all about phosphine, should probably begin with the words: “the flickering light that appears in the swamps (the famous “will-o’-the-wisps”) is the result of the spontaneous ignition of phosphine.” Well, the following definition is already encyclopedic: “phosphine, or hydrogen phosphorous (PH 3) is a colorless gas with an unpleasant odor (rotting fish, garlic or industrial carbide), poisonous, formed during the biochemical reduction of phosphoric acid esters, mainly in anaerobic conditions, i.e. without access to oxygen.”
Phosphorus compounds in nature
There are many other gaseous organophosphorus compounds found in nature, in the molecules of which the phosphorus atom P is connected to the carbon atom C. There are thousands of them. Many of them are part of ecosystems, including living cells of plants and microorganisms. The largest group of compounds with C-P bonds discovered about fifty years ago in living objects.
There are also phosphonates in soils - derivatives of organophosphorus compounds with preserved C-P bonds. True, there are not many of them, no more than 1-2% of the phosphorus contained in organic matter, so they cannot always be detected on arable land, but in swampy soils and meadows their content increases to 3-4%.
Under normal (aerobic) conditions, natural compounds of organic and mineral phosphorus are phosphates (orthophosphates). There are a great many of them. Organic phosphates are characterized by a C-O-P bond, in other words, carbon and phosphorus are connected through an oxygen atom.
One of the amazing mysteries of nature is that organic phosphates in living systems (for example, in algae and microorganisms) are synthesized and decomposed not arbitrarily, but according to the “golden ratio” rule, obeying a certain law described by famous nearby Fibonacci numbers (1, 1, 2, 3, 5, 8...), in which each next term is equal to the sum of the previous two. The harmony of nature is incomprehensibly manifested here in the accumulation and consumption of energy and matter (in particular, phosphorus) in ecosystems, described by the ratio that is approximately given by the classical “golden ratio” coefficient of 1.618 (5/3, 8/5, 13/8, etc.) etc.), i.e. 62% of the mentioned compounds should bind and accumulate and only 38% should be destroyed or volatilized. These patterns subsequently affect the accumulation of humus, the cycle of phosphorus and nitrogen, and the gaseous flows determined by emissions and “sinks” of carbon dioxide CO 2 , and the “respiration” of the soil (the release of CO 2 and the absorption of oxygen O 2). In fact, in nature there are fluctuations in the numerical values of this ratio in the range of 1.3-1.7. But, as has been noted more than once in the works of the author and other scientists, what is much more terrible is that the main reason for deviations and even violations of this pattern was anthropogenic activity.
Some experts have already drawn attention to the fact that new dangers may await us if this ratio tends to unity, i.e. accumulation and decomposition proceed with the same intensity, as happens, for example, in the carbon cycle, where due to “intervention” In the global economy, the ocean and biosphere currently absorb only half of carbon emissions (they should be 62%).
But let’s return to phosphine and its derivatives, in other words, to those organophosphorus compounds in which, together with phosphorus and carbon, various elements (nitrogen, sulfur, silicon, molybdenum, etc.) and their complexes are found. In conditions favorable for the growth of microorganisms (in particular, in swamps and tundra conditions with the observed warming), organophosphorus compounds are decomposed with the help of the enzyme (catalyst) C-P-lyase. Now it is found in 9 groups of bacteria that feed on phosphorus, extracting it from the breakdown of organophosphorus compounds. But fungi and yeast, which account for 50-70% of all microflora in ecosystems, do not break down these compounds. On the contrary, protozoa, mollusks and fungi synthesize them. Mushrooms can grow even at fairly high concentrations of phosphine, only their mycelium turns yellow.
Application, properties, dangers
Phosphine is poisonous (a dangerous concentration that can lead to death is 0.05 mg/l), and at a concentration of 2000 ml/m 3 (2 l/m 3, or 2·10 -3) it causes instant death. You have to deal with it first of all in agriculture when disinfecting granaries and protecting against ticks and other pests during transportation of crops, especially grain crops. Previously, it was actively used against rats and mice in barns. In Australia, they even resort to its help in the fight against rabbits that multiply too quickly. In addition, a number of herbicides and insecticides contain organophosphorus compounds based on phosphine and its derivatives. And finally, recently it has increasingly had to be dealt with in connection with the large-scale destruction of chemical weapons, which involves the neutralization of the poisonous organophosphorus compounds sarin and soman - phosphine derivatives.
Pure phosphine (without impurities) ignites at a temperature of 150°C, burns to form toxic phosphoric acid, but in the presence of impurities of diphosphine P 2 H 4 or gaseous phosphorus P 4 it can spontaneously ignite in air. The reaction of phosphine with oxygen (as well as the oxidation of similar methane - CH 4 and silane - SiH 4) is a branched chain reaction. chemical reactions, i.e. it proceeds faster and faster and can lead to an explosion. Phosphine oxidation occurs at room temperature, but the gas can be stable at low temperatures. The oxidation of phosphine can be accelerated by irradiating it with ultraviolet light. Its spontaneous ignition in air is possible at concentrations of 1.7-1.9% (17-19 l/m 3), or 26-27 g/m 3. So in bog ecosystems one often has to deal not only with the mentioned “will-o’-the-wisps,” but also with spontaneous combustion (by the way, common peat fires are of the same nature).
For fumigation (removing grain storage and agricultural products from mites and other pests), phosphides are usually used, in particular, phosphorus compounds with metals. Reacting with air moisture, phosphides release phosphine. Tablets and tapes containing phosphides are laid out in storage facilities at the rate of 9 g/t of grain or other products subject to long-term storage; they are even added to apples. It is believed that when ventilated, phosphine evaporates, although according to data available in the scientific literature, up to 13% of the toxic gas is absorbed in feed grain. Shouldn’t this circumstance alone force us to treat such “disinfection” with extreme caution?!
Currently, two compounds are approved for use for fumigation of grain during transportation and storage - methylbromine and methylphosphine, and the first is an order of magnitude less toxic (and effective) than the second. When using the latter, it is tacitly assumed that the poisonous phosphine, after being absorbed by the contents of the storage facility, is miraculously removed and evaporated, poisoning only ticks and other pests. It seems that previously it was not customary to think about how much this picture corresponds to reality. Meanwhile, almost half a century ago it was found that methylphosphine (a mixture of two gases - methane CH 4 and phosphine PH 3) is extremely toxic, almost like phosphine itself.
Methane and phosphine in the biosphere
It is no secret that methane released from swamps is considered one of the main greenhouse gases and remains the subject of active discussions and research in connection with the problems of global climate change. Alas, in Russia its concentration in the atmosphere is determined only at one weather station (Teriberka on the Kola Peninsula). But it wouldn’t hurt to measure it over Siberian swamps!
As is known, huge reserves of methane are preserved in the depths of the earth (7·10 11 -3·10 13 tons), and 4·10 11 tons of them are in the Arctic permafrost zone. On land, methane is contained in organic compounds of swamps, sediments and detritus, and in the World Ocean - in gas hydrates lying under the bottom under conditions of low temperatures. In the UN Climate Change Report, experts report that in Siberia, methane emissions from swamps and permafrost in last years is growing rapidly. The maximum emission of methane from tundra soils is achieved at 8-10°C, and at 5°C its oxidation to CO 2 and water predominates. It is formed in all soil horizons. As a result of recent studies, it turned out that, for example, our southern shrub tundra (the vicinity of Vorkuta) served as a carbon sink for only two years out of the last five.
This is a rather dangerous trend, especially when you take into account that our country accounts for 2/3 of all swamps on Earth. Our area of wetlands exceeds the area of all agricultural land: according to data for 2003, 343 million hectares of wetlands (of which 130 million hectares are not covered with forest) and 221 million hectares of agricultural land (of which 123 million hectares are arable).
And here is how MSU employees assessed the release of methane in 2007 based on the results of measurements in swamps in the Tomsk region. According to their estimates, the average methane flux was about 10 mg/m2 per hour. IN summer period 2.4 kg/ha can be released per day, 432 kg/ha per season (6 months). And from 130 million hectares of swamps - almost 60 million tons. The oxidation of such an amount of methane will require twice as much oxygen - 120 million tons.
The main “side” effect of methane release should be recognized as the fact that in tundra and swamp ecosystems at low temperatures, methane not only represents a significant carbon reserve that can significantly change its content in the atmosphere, but is also closely related to phosphorus organic compounds, which are invariably present in plants, microflora of swamps and sediments (mainly due to the mentioned C-P connection). And its release from those places where it was previously synthesized, due to the intensification of biochemical fermentation processes with increasing temperature, occurs not least due to the decomposition of phosphine-based compounds. In other words, the emission of CH 4 and PH 3 gases occurs in parallel. Meanwhile, ecologists and climatologists only monitor changes in the content of CO 2 and CH 4 in the atmosphere, and no one takes into account the content of pH 3. But in vain!
This omission is explained, in part, by the fact that only a few specialists are aware of methods that make it possible to measure the content of phosphorus in the gaseous state in the atmosphere. After all, even in scientific world There is still an opinion that phosphorus in nature exists mainly in the form of phosphates and after hydrolysis of the P-O-P, P-O-C and even P-C bonds it turns into a solid. Phosphorus fluxes into the atmosphere in the form of volatile compounds such as PH 3 are considered negligible and are neglected. Determining the content of phosphorus released into the atmosphere with phosphine using only the usual methods used to detect phosphorus in solid compounds noticeably distorts the real picture of the phosphorus cycle in ecosystems. At the same time, the appearance of toxic and spontaneously combustible phosphine in the atmosphere is ignored.
Phosphine threat: simple assessments
Meanwhile, the simplest quantitative assessment of phosphine release in ecosystems can be obtained by studying areas flooded with water, simulating flooded meadows or rice paddies. As was established in a study conducted back in 1926 at the Moscow Agricultural Academy. K. A. Timiryazev, in a series of six experiments carried out under strictly controlled conditions, 9.7 mg of phosphorus from 1 kg of soil is converted into gas form (phosphine) per hour. A not too complicated calculation gives 2.13 kg/ha per day. But this is almost the same amount of methane released from swamps! Therefore, during the season we get 383 kg/ha, and from the entire area of treeless swamps (130 million hectares) - about 50 million tons of pH 3 . For its oxidation to phosphoric acid according to the formula
PH 3 + 2O 2 → H 3 PO 4
it will be necessary, as is easy to see, twice as much oxygen - almost 100 million tons (for methane these values were 60 and 120 million tons, respectively).
Indirect confirmation of the release of phosphine from soils is also provided by studies of phosphorus flows in rice paddies - from planting to harvesting, phosphorus losses in flooded soils are 3-8 times higher than its content in grain and straw. The maximum removal of P 2 O 5 reaches 100 kg/ha. 4 times more phosphorus is removed from soils than is stored in plants. Total losses phosphorus from the top (20 cm) soil layer, according to various estimates, is 960-2940 kg/ha. There is evidence that when rice is grown on flooded fields for 32 years, more than half of the humus is lost from the soil, and with it, of course, nitrogen and phosphorus are carried away.

This can also occur due to the release of their gaseous different forms- ammonia (NH 3) and phosphine (PH 3). It has long been known that in terms of their chemical properties they are chemical structural analogues. I repeat, defining phosphorus and nitrogen only in mineral form and ignoring gas components does not reflect the true processes in ecosystems, especially under anaerobic conditions. In particular, direct confirmation that in swamp ecosystems phosphorus is also released along with methane was obtained in recent studies.
Returning to the discussion about a possible underestimation of the phosphine content in the atmosphere, it should be noted that a quite significant contribution can be made not only by the swamps of the North or the tropics, but also by vast rice plantations (primarily in India, China, Japan and the countries of Southeast Asia).
In the scientific literature there is evidence that up to 3.5 kg/ha of phosphorus falls on the ground with precipitation. In other words, this is only about 1% of the phosphorus that is estimated to be lost from wetland systems or phosphine-flooded soils to the atmosphere (383 kg/ha), the remaining 99% appears to be rapidly oxidized, precipitated or degraded (e.g. as a result of hydrolysis) in the ground layers of air, lithosphere and biosphere, ensuring the redistribution of phosphorus on the surface of the earth.
Of course, phosphine, like methane, exists in the atmosphere, but it must be admitted that the phosphorus cycle has been studied much less well than the nitrogen or carbon cycle. Highly active phosphorus compounds in the presence of oxygen quickly transform into neutral complexes, “harmless” phosphates. In addition, phosphorus is usually scarce in ecosystems, meaning it is present in low concentrations. Therefore, I repeat, attempts to take into account phosphorus only in the form of phosphates can lead to a noticeable distortion of its true role in ecosystems. And what underestimation of this role can lead to can be clearly seen, for example, in previously thoughtlessly drained swamps, which easily ignite in dry years due to methane (CH 4), silane (SiH 4) and phosphine (PH 3).
Based on the results of measurements at the Teriberka weather station mentioned above, it was established that in 1990, 48.8 million tons of methane were released into the atmosphere from the territory of Russia (remember, our estimates for the entire area of treeless swamps were about 60 million tons). For 1996-2003 the highest concentration was recorded in 2003. This year was the warmest for all of Russia, and this was especially true for summer and autumn in swamp and tundra areas (Yakutia, Western Siberia) - on average, the temperature here was almost 6°C higher than the long-term one. Under these conditions, a summer decrease in the content of high-level ozone O 3 over the North of Russia by 5-10% was simultaneously observed. But in summer, here too, the processes of photosynthesis and oxygen formation accelerate. Therefore, it is obvious that ozone was intensively consumed here to oxidize the increased amount of methane and phosphine under the warm conditions of 2003.
From phosphine to oxygen: some statistics and philosophy
It is no secret that, due to its rich biological resources, Russia is already considered a global donor of oxygen. According to experts, 8130 million tons of O 2 are formed annually over its territory. It seems that we will not be too far from the truth if we assume that the process of photosynthesis, which is responsible for the formation of this mass of oxygen, obeys the mentioned “law of universal harmony” - the rule of the “golden section”. After all, the formation of 1 ton of organic matter during photosynthesis requires 1.47 tons of carbon dioxide, 0.6 tons of water and 3.84 Gcal of solar energy, and at the same time 1.07 tons of oxygen are released. The ratio between the amount of absorbed CO 2 and released O 2 (1.47: 1.07) is not so different from the “golden” one.
According to some published estimates, oxygen consumption in Russia (breathing, fuel combustion and other industrial needs) is 2784 million tons. Then its “production” by Russia exceeds its consumption by 5346 million tons. But in other calculations, which take into account oxygen consumption by microflora (formerly total soil) for “respiration”, the Russian excess of oxygen production over its consumption is already an order of magnitude lower - 560 million tons. Meanwhile, as some researchers believe, the “respiration” of the soil is regulated by its “golden ratio” rule, which determines the ratio of carbon dioxide released by microflora gas and oxygen consumed. On virgin lands, the value of this value is close to 1.58, and on arable land it ranges from 1.3-1.75 - in other words, oxygen is consumed “economically” (42-37%) in the process of “breathing” the soil, and carbon dioxide is released more (58-63%). If we proceed from the average value of the “golden section” of 1.52 for the CO 2: O 2 ratio, then with the emission of CO 2 from Russian soils of 10,409 million tons of oxygen, another 6,848 million tons are consumed for the “breathing” of Russian soils (2004 estimates based on data employees of the Institute of Fundamental Problems of Biology of the Russian Academy of Sciences, in particular V. N. Kudeyarov).
A kind of “golden proportion” is also observed between CO 2 runoff and its emission on the Russian scale. The ratio between the runoff, amounting to 4450 million tons per year (in terms of carbon), and emissions (2800 million tons - in the same units) turns out to be equal to 1.59, i.e. surprisingly close to the “golden” one. Well, while there is no excess CO 2 over Russia as a whole, our ecosystems absorb more than we emit, our forests save us and cover our “sins”. But in recent years (primarily in the North), it has been increasingly noted that ecosystems are not coping with the “plan” for absorption and the noted ratio is violated.
However, it is much more important that, as follows from a number of estimates, in Russia the total consumption of oxygen per year for our needs (2784 million tons), soil respiration (6848 million tons) and oxidation of methane and phosphine (220 million tons) is approaching 10 billion tons, which is almost 2 billion tons more than all our forests produce. And this sad balance seems to me to be a much more serious problem than the expected quota trading. For the sake of preservation environment and the biosphere of the planet, whose resources we currently consume 25% more than they have time to recover, we must finally realize that without limiting consumption, we and our descendants simply cannot survive. And not least of all, this concerns oxygen. There seems to be a lot of it in the atmosphere (21%), but it should not be allowed that more of it is consumed on Earth than is produced.
Summing up
It is no secret that over the past 100 years, as a result of thoughtless human activity and ignorance of the laws of nature, carbon dioxide emissions into the atmosphere (and its content there), according to various estimates, have increased by 25-35%. One of the poorly calculated consequences global warming there may be a sharp intensification of biochemical processes in natural areas swamps and permafrost. At the same time, the release of not only methane (this is almost obvious) may sharply increase, but also gases that have been little studied in terms of their influence on the biosphere: ammonia, silane and phosphine, which will require a lot of oxygen for oxidation and neutralization. But there are also feedback effects that have not been fully analyzed (for example, more intense methane release will accelerate the further increase in CO 2 concentration in the atmosphere, which, in turn, can lead to a sharp slowdown in photosynthesis). As follows from recent studies, in the 90s of the last century the compensating role of photosynthesis in boreal forests noticeably weakened. But previously it was firmly established that trees at all latitudes reliably contributed to photosynthesis and CO 2 assimilation. Dangerous trend! And examples of such “metamorphoses” of forests are multiplying from year to year.

At present, we know almost nothing about the isolation and oxidation of silane (SiH 4), which was mentioned more than once in this article. Meanwhile, all marsh plants, cereals and microorganisms are rich in organic silicon. The peat of high bogs contains 43% SiO 2, transitional peat - 28%, lowland peat - 21%. So far, there is only fragmentary evidence that silane in combination with phosphine forms insufficiently studied complexes - silylphosphines. The processes of silane release, its oxidation and combination with other elements require serious study.
And in conclusion - a fantastic-looking plot that should make everyone who has not yet lost this ability think. In the ground layer of the atmosphere, due to the rapid increase in the content of carbon dioxide and some other “dead” gases, in the foreseeable future there may be a shortage of oxygen not only due to a slowdown in photosynthesis, increased consumption for oxidation, combustion and respiration, but also due to the “screen” poisonous gases that interfere with the flow of O 2 from more high strata atmosphere.
For billions of years, the basis of all life on Earth was photosynthesis, which regularly supplied the planet with oxygen. Alas, as some researchers rightly note, modern civilization, for the first time in history, seems to have managed to slow down the replenishment of the atmosphere with oxygen, and brought nature to the point of bifurcation. Will she survive?
See, for example: Eldyshev Yu.N. Is methane the culprit behind global warming? // “Ecology and Life”, 2007, No. 11, p. 45; Climate change: facts and factors // “Ecology and Life”, 2008, No. 3, p. 44.
See, for example, the article by Kravchenko I.K. in the journal “Microbiology”, No. 6, 2007.
Industrial exposure occurs when PH3 is used in the production of acetylene or when phosphine is used as an additive in the production of silicone crystals. Aluminum phosphide, used as a grain fumigant, and zinc phosphide, used as a rodenticide, release phosphine gas when exposed to moisture, which can be fatal.
This garlic-like gas causes severe gastrointestinal symptoms. IN severe cases Coma, convulsions, hypotension and pulmonary edema develop. Unlike arsine gas, phosphine does not cause hemolytic anemia.
A) Phosphine Gas Poisoning Clinic. Phosphine gas causes gastrointestinal symptoms and disturbances in the respiratory, cardiovascular and central nervous systems due to metabolic changes.
Significant clinical manifestations of acute phosphine exposure include headache, fatigue, nausea, vomiting, cough, dyspnea, paresthesia, jaundice, ataxia, intention tremor, weakness and diplopia. In fatal cases, autopsy revealed centrilobular liver necrosis, congestive heart failure with pulmonary edema, and focal myocardial necrosis.
b) Treatment of phosphine gas poisoning:
- Pre-hospitalization measures in the contagion zone or Decon area. After overexposure, small amounts of phosphine remain in the victim's clothing, which are not sufficient to pose a hazard to medical personnel outside the contaminated area.
1. Rescuers must be provided with fully self-contained respirators, special protective clothing and gloves.
2. It is necessary to quickly assess the patency of the airways, the state of breathing and blood circulation, ensure stability of the spine (if injury is suspected), ensure patency of the airways and adequate breathing, and provide additional oxygen.
3. Spray the victim with water from a hose and, if there is a possibility of gas accumulation in clothing (for example, in the case of prolonged exposure in a closed room), remove the clothing and pack it in a double-layer bag.
- Treatment in hospital:
1. Investigate and ensure adequate functioning of the airways, breathing and circulation.
2. In case of respiratory distress, use an oxygen mask.
3. Control heart rate; do with 12 leads. In case of severe exposure, exclude myocardial infarction.
4. Laboratory tests: hematocrit, electrolytes, blood urea nitrogen and/or creatinine, liver enzymes, Ca, Mg and blood gases. Request for other laboratory tests should be made.
5. Treat pulmonary edema. Symptoms may not appear for 72 hours.
6. Liver damage may become obvious after 2-3 days.
Nearest source of stone containing phosphine, was indicated on the maps, and David sent a working group of blue and green horsemen there to begin preparing the firestone.
Now they knew all the tricks of the enemy, they learned to evaluate the characteristics of attacks, learned how to conserve the strength of horsemen and animals, how to protect themselves from fumes phosphine and blows of Threads.
Fire jets phosphine, erupted by the dragons, formed a continuously changing pattern of light in the air.
The horsemen discovered deposits phosphine on a plateau somewhere between the Malay River and Sadrid.
While the dragon positioned his bulky body on such an unsuitable landing site, his wide wings drove the smelling scent along the yard phosphine air.
Then he washed off the stinking phosphine pants and a shirt and dried them in the sun, hanging them in the bushes.
When Jaxom entered his room on his way to change the stinking phosphine flight suit, he caught his eye on the sketch of the bay, still laid out on the work table.
Jaxom shoved his portion into Ruth's mouth and, as always, experiencing inner trepidation, began to listen to how the dragon's powerful teeth crushed the rich phosphine stone.
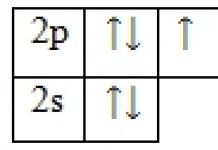
Oxidation state at PH3
General information about phosphine and oxidation state in PH3
The gross formula is PH3 (the structure of the molecule is shown in Fig. 1). Molar mass phosphine is 34.00 g/mol.
Meaning of the word phosphine
1. The structure of the phosphine molecule, indicating the bond angle and length chemical bond.
At low temperatures it forms solid clarate 8PH3×46H2O. Density - 1.5294 g/l. Boiling point - (-87.42oC), melting point - (-133.8oC).
In ORR it is a strong reducing agent, oxidized by concentrated sulfuric and nitric acids, iodine, oxygen, hydrogen peroxide, sodium hypochlorite. The donor properties are much less pronounced than those of ammonia.
PH3, oxidation states of elements in it
To determine the oxidation states of the elements that make up phosphine, you first need to understand for which elements this value is precisely known.
Phosphine is the trivial name for phosphorus hydride, and, as is known, the oxidation state of hydrogen in hydrides is (+1). To find the oxidation state of phosphorus, we take its value as “x” and determine it using the electrical neutrality equation:
x + 3×(+1) = 0;
This means the oxidation state of phosphorus in phosphine is (-3):
Examples of problem solving

3. Molecules. Chemical bond. Structure of substances
Chemical particles formed from two or more atoms are called molecules(real or conditional formula units polyatomic substances). Atoms in molecules are chemically bonded.
Chemical bonding refers to the electrical forces of attraction that hold particles together. Every chemical bond in structural formulas seems valence line For example:
H–H (bond between two hydrogen atoms);
H3N – H+ (bond between the nitrogen atom of the ammonia molecule and the hydrogen cation);
(K+) – (I-) (bond between potassium cation and iodide ion).
A chemical bond is formed by a pair of electrons ( ), which in the electronic formulas of complex particles (molecules, complex ions) is usually replaced by a valence feature, in contrast to the own, lone electron pairs of atoms, for example:

The chemical bond is called covalent, if it is formed by sharing a pair of electrons with both atoms.
In the F2 molecule, both fluorine atoms have the same electronegativity, therefore, the possession of an electron pair is the same for them. Such a chemical bond is called nonpolar, since each fluorine atom electron density is the same in electronic formula molecules can be conditionally divided equally between them:
In the hydrogen chloride molecule HCl, the chemical bond is already polar, since the electron density on the chlorine atom (an element with higher electronegativity) is significantly higher than on the hydrogen atom:
A covalent bond, for example H–H, can be formed by sharing the electrons of two neutral atoms:
H · + · H > H – H
H H
This mechanism of bond formation is called exchange or equivalent.
According to another mechanism, the same covalent H – H bond occurs when the electron pair of the hydride ion H is shared by the hydrogen cation H+:
H+ + (:H)- > H – H
H H
The H+ cation in this case is called acceptor a anion H – donor electron pair. The mechanism of covalent bond formation will be donor-acceptor, or coordination.
Single bonds (H – H, F – F, H – CI, H – N) are called a-bonds, they determine the geometric shape of molecules.
Double and triple bonds () contain one?-component and one or two?-components; The ?-component, which is the main one and conditionally formed first, is always stronger than the ?-components.
The physical (actually measurable) characteristics of a chemical bond are its energy, length and polarity.
Chemical bond energy (E sv) is the heat that is released during the formation of a given bond and is spent on breaking it. For the same atoms, a single bond is always weaker than a multiple (double, triple).
Chemical bond length (lсв) – internuclear distance. For the same atoms, a single bond is always longer, than a multiple.
Polarity communication is measured electric dipole moment p– the product of the real electric charge (on the atoms of a given bond) by the length of the dipole (i.e.
Phosphorus. Phosphine
communication length). The larger the dipole moment, the higher the polarity of the bond. Real electric charges on atoms in a covalent bond is always less in value than the oxidation states of the elements, but coincide in sign; for example, for the H+I-Cl-I bond, the real charges are H+0'17-Cl-0'17 (bipolar particle, or dipole).
Molecular polarity determined by their composition and geometric shape.
Non-polar (p = O) will be:
a) molecules simple substances, since they contain only non-polar covalent bonds;
b) polyatomic molecules complex substances, if their geometric shape symmetrical.
For example, CO2, BF3 and CH4 molecules have the following directions of equal (in length) bond vectors:

When adding bond vectors, their sum always goes to zero, and the molecules as a whole are nonpolar, although they contain polar bonds.
Polar (p> O) will be:
A) diatomic molecules complex substances, since they contain only polar bonds;
b) polyatomic molecules complex substances, if their structure asymmetrically, that is, their geometric shape is either incomplete or distorted, which leads to the appearance of a total electric dipole, for example, in the molecules NH3, H2O, HNO3 and HCN.
Complex ions, such as NH4+, SO42- and NO3-, cannot be dipoles in principle; they carry only one (positive or negative) charge.
Ionic bond occurs during the electrostatic attraction of cations and anions with almost no sharing of a pair of electrons, for example between K+ and I-. The potassium atom has a lack of electron density, while the iodine atom has an excess. This connection is considered extreme a case of a covalent bond, since the pair of electrons is practically in the possession of the anion. This connection is most typical for compounds of typical metals and non-metals (CsF, NaBr, CaO, K2S, Li3N) and substances of the salt class (NaNO3, K2SO4, CaCO3). All these compounds at room conditions are crystalline substances, which are collectively called ionic crystals(crystals built from cations and anions).
Another type of connection is known, called metal bond, in which valence electrons are so loosely held by metal atoms that they actually do not belong to specific atoms.
Metal atoms, left without external electrons clearly belonging to them, become, as it were, positive ions. They form metal crystal lattice. The set of socialized valence electrons ( electron gas) holds positive metal ions together and at specific lattice sites.
In addition to ionic and metallic crystals, there are also atomic And molecular crystalline substances in whose lattice sites there are atoms or molecules, respectively. Examples: diamond and graphite are crystals with an atomic lattice, iodine I2 and carbon dioxide CO2 (dry ice) are crystals with a molecular lattice.
Chemical bonds exist not only inside the molecules of substances, but can also form between molecules, for example, for liquid HF, water H2O and a mixture of H2O + NH3:

Hydrogen bond is formed due to the forces of electrostatic attraction of polar molecules containing atoms of the most electronegative elements - F, O, N. For example, hydrogen bonds are present in HF, H2O and NH3, but they are not in HCl, H2S and PH3.
Hydrogen bonds are unstable and break quite easily, for example, when ice melts and water boils. However, some additional energy is spent on breaking these bonds, and therefore the melting temperatures (Table 5) and boiling points of substances with hydrogen bonds

(for example, HF and H2O) are significantly higher than for similar substances, but without hydrogen bonds (for example, HCl and H2S, respectively).
Many organic compounds also form hydrogen bonds; Hydrogen bonding plays an important role in biological processes.
Examples of Part A tasks
1. Substances with only covalent bonds are
1) SiH4, Cl2O, CaBr2
2) NF3, NH4Cl, P2O5
3) CH4, HNO3, Na(CH3O)
4) CCl2O, I2, N2O
2–4. Covalent bond
2. single
3. double
4. triple
present in the substance
5. Multiple bonds exist in molecules
6. Particles called radicals are
7. One of the bonds is formed by a donor-acceptor mechanism in a set of ions
8. Most durable And short bond - in a molecule
9. Substances with only ionic bonds - in the set
10–13. Crystal lattice of matter
1) metal
3) atomic
4) molecular
Phosphorus compounds.
R-3. Metal phosphides are ionic covalent compounds. Phosphides of s-metals (except Be) and lanthanides are ionic salt-like compounds; they are easily hydrolyzed by water and acids: Mg3P2 + 6H2O = 3Mg(OH)2↓ + 2PH3 Na3P + 3HCl = 3NaCl + PH3. Phosphides of d-elements are metal-like chemically inert compounds. The exception is phosphides of metals of groups I and II, side subgroups, which are also salt-like, but with a large admixture of covalence. Phosphorus does not form stable compounds with antimony, bismuth, lead and mercury.
The compound of phosphorus and hydrogen is called hydrogen phosphide, although the electronegativity of these elements is almost equal. The compound has the formula PH3 and is called phosphine. This is an extremely poisonous gas with an unpleasant garlic odor, boiling point = -88°C. There are no hydrogen bonds between phosphine molecules in liquid and between water and phosphine molecules during dissolution, therefore the boiling point is low and phosphine practically does not dissolve in water. The molecule is a pyramid with a phosphorus atom at the apex and an angle of 93.5° between the P-H bonds, which indicates the absence of hybridization of the phosphorus atomic orbitals during the formation of this compound. The bonds are formed by almost pure p-orbitals. The lone electron pair of phosphorus remains in the 3s orbital, so phosphine is weak foundation and a weak complexing agent in general. The phosphonium cation is formed only with the strongest acids in an anhydrous environment (HJ, HClO4, HBF4), for example PH3 + HJ = PH4J. Water easily decomposes phosphonium salts. Phosphine exhibits strong reducing properties: PH3 + 2O2 = H3PO4 (at 150°C this reaction occurs explosively), PH3 + 6AgNO3 + 3H2O = 6Ag↓ + H2(PHO3) + 6AgNO3 PH3 + 3J2 + 3H2O = H2(PHO3) + 6HJ . The synthesis of phosphine from simple substances cannot be carried out, since the P-H bond is not strong enough due to its length and due to the insignificant contribution of the electrostatic component. Therefore, phosphine is obtained by hydrolysis of metal phosphides or dissolution of phosphorus in alkali (reactions given above).
The main compounds of phosphorus in its positive oxidation states are oxides, oxygen-containing acids and halides. It is advisable to consider them separately.
Phosphorus oxides– Р4О6 and Р4О10 are acidic oxides, have a molecular structure, are solids (tmelt(Р4О6)=23.8°С, the molecular modification Р4О10 sublimes at 3590С, and the polymer modification melts at 580°С), both dissolve in water, giving hydroxides, which are acids, phosphorous and orthophosphoric, respectively. Phosphorus (V) oxide is very hygroscopic, absorbs moisture from the air, therefore it is used as a desiccant, and also as a water-removing agent: P2O5 + HNO3 = HPO3 + N2O5, which produces metaphosphoric acid or polyphosphoric acids - (HPO3)3-4. Phosphorus (III) oxide, in which phosphorus is in an intermediate oxidation state, is capable of further oxidation reactions and disproportionation reactions, for example: P4O6 + 2O2 = P4O10 P4O6 + 6H2O (hor) = 3H3PO4 + PH3, at 210°C in an H2 atmosphere the reaction 5P4O6 = 2P4 + 3P4O10 occurs. Phosphorus (V) oxide does not have oxidizing properties, but can itself be obtained by the oxidation of phosphorus under anhydrous conditions, for example, during the thermal decomposition of certain salts: 6P + 5KClO3 = 3P2O5 + 5KCl
Oxygen acids of phosphorus. The variety of oxygen acids of phosphorus is caused by the following reasons: 1. The valence of phosphorus can be III or V. 2. In the case of valency V, the formation of ortho and meta acids is possible, differing in the number of attached water molecules. 3. In all hydroxides, phosphorus exhibits coordination number 4, such hydroxides are more stable for it; if there are not enough oxygen atoms, then a P-H bond is formed ((HO)2PHO, and not P(OH)3, etc.). 4. Phosphoric acids tend to form linear or cyclic polymers. 5. Under certain conditions, the formation of a P-R bond is possible. 6. As with all hydroxides, further oxidation results in the formation of peroxoacids. Let us present the structure and properties of the most famous phosphorus acids.
H3PO4 – orthophosphoric acid. It is a tribasic acid, medium in dissociation in the first step (Ka = 7.52.10-3) and weak in the other two steps. In the anhydrous state it forms transparent hygroscopic crystals with melting point = 42°C. It dissolves in water in any concentration. Orthophosphoric acid is obtained by dissolving phosphorus (V) oxide in water, by burning phosphine, by oxidizing any forms of phosphorus in an acidic environment, by hydrolyzing binary compounds of phosphorus (V): P4S10 + 16H2O = 4H3PO4 + 10H2S. The industry uses the method of burning phosphorus with subsequent dissolution of the oxide, as well as the displacement of orthophosphoric acid from calcium phosphate with concentrated sulfuric acid when heated: Ca3(PO4)2 + 3H2SO4 = 3CaSO4↓ + 2H3PO4. This acid corresponds to three series of salts - medium (phosphates or orthophosphates) and acidic (hydrogen phosphates and dihydrogen phosphates). Phosphates and hydrophosphates of all metals, except sodium, potassium, rubidium and cesium, are insoluble in water. Dihydrogen phosphates are soluble. Soluble phosphates undergo strong hydrolysis at the anion; the highest hydrolysis constant is characterized by the phosphate anion, and the smallest by dihydrogen phosphate. Hydrolysis at the anion leads to an alkaline environment of salt solutions. Acid anions, simultaneously with hydrolysis, participate in dissociation equilibrium, which leads to an acidic solution environment, for dihydrogen phosphate to a greater extent, for hydrogen phosphate to a lesser extent. As a result of these processes, the sodium dihydrogen phosphate solution has a weakly acidic environment, the hydrogen phosphate solution has a weakly alkaline environment, and the phosphate solution has a strongly alkaline environment. Ammonium phosphate, as a salt formed by a weak acid and base, is completely decomposed by water. Orthophosphates melt without decomposition at very high temperatures. Hydrophosphates give diphosphates when heated: 2K2HPO4 = K4P2O7 + H2O. When heated, dihydrogen phosphates turn into polymetaphosphates: xKH2PO4 = (KPO3)x + H2O. Phosphates do not have strong oxidizing properties, but can be reduced by carbon when heated. In the presence of silicon dioxide, this reaction leads to the production of phosphorus (the reaction equation was given); in the absence of SiO2, the process proceeds as follows: Ca3(PO4)2 + 8C = Ca3P2 + 8CO. Heating ammonium phosphate leads to the gradual loss of ammonia molecules, eventually forming polymetaphosphoric acid at temperatures above 300°C.
When orthophosphoric acid is dehydrated, condensed phosphoric acids are formed, which contain one or more bridging oxygen atoms. In this case, chain, cyclic and mixed structures are formed. Let's look at the simplest of them.
Diphosphoric (pyrophosphoric) acid – H4P2O7. It is obtained by heating orthophosphoric acid to 2000C. In the anhydrous state, it is colorless crystals with melting point = 61°C, which are highly soluble in water with the formation of a much stronger acid than phosphoric acid. This acid is especially strong in the first two stages. Any condensed acid is stronger than a single acid, since its dissociation produces a more stable anion. Solutions of pyrophosphoric acid are unstable, since a gradual addition of a water molecule occurs to form two molecules of orthophosphoric acid. More stable salts are pyrophosphates, which, as already mentioned, can be obtained by heating hydrogen phosphates.
Metaphosphoric acids – (HPO3)x, where x=3,4,6. Cyclic condensed acids containing a cycle of alternating phosphorus and oxygen atoms. They are obtained by dissolving phosphorus (V) oxide in orthophosphoric acid, as well as by heating pyrophosphoric acid to 300°C: 3H4P2O7 = 2(HPO3)3 + H2O. All metaphosphoric acids are very strong, for trimetaphosphoric acid Ka2 = 0.02. All these acids also gradually transform into orthophosphoric acid in aqueous solution. Their salts are called tri-, tetra- and hexametaphosphates, respectively.
By oxidation of phosphorus (V) oxide one can obtain peroxophosphoric acid: P4O10 + 4H2O2 + 2H2O = 4H3PO5.
Phosphoric (hypophosphoric) acid H4P2O6 has a R-R connection. Structural formula can be represented as (OH)2OR-PO(OH)2.
Properties of phosphine
From the formula it is clear that the valency of phosphorus is 5, and the oxidation state +4 is a formal value associated with the presence of bonds between identical atoms. This is a tetrabasic acid, the strength of which corresponds to phosphoric acid. It is obtained by the reaction: PbP2O6 + 2H2S = 2PbS↓ + H4P2O6 and is released from solution in the form of a dihydrate with melting point = 62°C. In an acidic solution it disproportionates into orthophosphoric and phosphorous acids.
Phosphorous acid H3PO3 or H2. This is a dibasic acid of medium strength, in the anhydrous state it is a solid with a melting point of 74°C. It is obtained by the hydrolysis of phosphorus (III) halides, as well as by the oxidation of white phosphorus with chlorine under water: P4 + 6Cl2 + 12H2O = 4H2 + 12HCl. As mentioned above, the compound of composition P(OH)3 is less stable, therefore isomerization occurs with the formation communications RN, which no longer dissociates in an aqueous solution. Salts of phosphorous acid are called phosphites, acid salts are called hydrophosphites. Most phosphites (except alkali metal salts) are insoluble in water. Like all phosphorus (III) compounds, phosphorous acid is a strong reducing agent; it is oxidized to phosphoric acid by halogens, nitrogen dioxide and other oxidizing agents, and also reduces low-active metals from a solution of their salts, for example: HgCl2 + H2 + H2O = H3PO4 + 2HCl + Hg↓. When heated, it disproportionates: 4H2 = 3H3PO4 + PH3.
Phosphorous (phosphinic) acid H3PO2 or H. This is a solid substance with melting point = 26.5°C, the aqueous solution of which is a fairly strong (Ka = 7.9 .10-2) monobasic acid. The phosphorus in this compound also has five bonds, two of which are with hydrogen atoms. Only undergoes dissociation N-O connection. The formal oxidation state of phosphorus in this compound is +1. Hypophosphorous acid and its salts, hypophosphites, are strong reducing agents. Metal cations, even those in the voltage series before hydrogen, are capable of being reduced to metal: NiCl2 + Na + 2H2O = H3PO4 + HCl + NaCl + H2+ Ni↓. When heated, phosphorous acid disproportionates: 3H = PH3 + 2H2. As the temperature increases, phosphorous acid has also been shown to decompose into phosphoric acid and phosphine. Hypophosphites of alkali and alkaline earth metals are obtained by the interaction of phosphorus and alkali (see above). Oxidation of phosphine with a mild oxidizer: PH3 + SO2 = H + S↓ (catalysts - mercury and traces of water).
Phosphorus halides PX3 and PX5. All phosphorus halides are known except PJ5. In the case of phosphorus (III), these are pyramidal molecules with a phosphorus atom at the top and with angles between R-X connections, equal to 100°. Phosphorus(V) halides are trigonal bipyramids with sp3d hybridization of phosphorus atomic orbitals. Both phosphorus fluorides are gases under normal conditions, PCl3 and PBr3 are liquids, and triiodide, pentachloride and pentabromide are solids. The last two compounds are salts with complex ions PCl5: +-, PBr5: +Br-. When heated, both compounds split off a halogen molecule and transform into a trihalide. Phosphorus halides are obtained by direct synthesis. Only PF3 – indirectly: PCl3 + AsF3 = PF3 + AsCl3. All phosphorus halides are subject to hydrolysis, and trihalides are also capable of oxidation: 2PCl3 + O2 = 2POCl3 - phosphorus oxychloride, can also be obtained by other reactions: PCl3 + 2CrO3 = POCl3 + Cr2O3↓ + O2, 6PCl5 + P4O10 = 10POCl3. Trihalides also add sulfur : PCl3 + S = PSCl3. In non-aqueous solutions the following reactions are possible: KF + PF5 = K HF(liquid) + PF5 = H – hexafluorophosphoric acid, stable only in aqueous solution, comparable in strength to perchloric acid.
Previous567891011121314151617181920Next
SEE MORE:
Phosphine. Phosphorus oxides and phosphoric acids: properties, preparation.
The word phosphine
Medical and biological significance of phosphorus.
Phosphine (hydrogen phosphide, phosphorus hydride, according to IUPAC nomenclature - phosphane PH3) is a colorless, very poisonous, rather unstable gas (at normal conditions) with a specific smell of rotten fish.
Physical properties
Colorless gas. It dissolves poorly in water and does not react with it. At low temperatures it forms solid clathrate 8РН3·46Н2О. Soluble in benzene, diethyl ether, carbon disulfide. At −133.8 °C it forms crystals with a face-centered cubic lattice.
The phosphine molecule has the shape of a trigonal pyramid with molecular symmetry C3v (dPH = 0.142 nm, HPH = 93.5o). The dipole moment is 0.58 D, significantly lower than that of ammonia. The hydrogen bond between PH3 molecules is practically not observed and therefore phosphine has lower melting and boiling points.
]Receipt
Phosphine is obtained by reacting white phosphorus with hot alkali, for example:
It can also be obtained by treating phosphides with water or acids:
When heated, hydrogen chloride reacts with white phosphorus:
Decomposition of phosphonium iodide:
Decomposition of phosphonic acid:
or restoring it:
Chemical properties
Phosphine is very different from its counterpart ammonia. Its chemical activity is higher than that of ammonia; it is poorly soluble in water, as a base is much weaker than ammonia. The latter is explained by the fact that the H-P bonds are weakly polarized and the activity of the lone pair of electrons in phosphorus (3s2) is lower than that of nitrogen (2s2) in ammonia.
In the absence of oxygen, when heated, it decomposes into elements:
spontaneously ignites in air (in the presence of diphosphine vapor or at temperatures above 100 °C):
Shows strong restorative properties:
When interacting with strong proton donors, phosphine can produce phosphonium salts containing the PH4+ ion (similar to ammonium). Phosphonium salts, colorless crystalline substances, are extremely unstable and easily hydrolyze.
Phosphine salts, like phosphine itself, are strong reducing agents.
Toxicity
Phosphine is highly toxic and affects nervous system, disrupts metabolism. MPC = 0.1 mg/m³. The odor is noticeable at a concentration of 2-4 mg/m³; prolonged inhalation at a concentration of 10 mg/m³ is fatal. In human blood, the phosphine content is no more than 0.001 mg/m³.
The following phosphorus oxides are known:
Phosphorus(III) oxide - binary inorganic compound, phosphorus oxide with the formula P4O6, white flakes or crystals with an unpleasant odor, react with water.
Receipt
- Careful oxidation of white phosphorus with nitrous oxide or carbon dioxide:
- Reverse disproportionation of phosphorus(V) oxide and white phosphorus:
[edit]Physical properties
Phosphorus(III) oxide forms white flakes or crystals with an unpleasant odor.
It dissolves well in organic solvents (benzene, carbon disulfide).
Unstable in the light, first turns yellow and then red.
Properties
P4O10 interacts very actively with water (the H-form absorbs water even explosively), forming mixtures of phosphoric acids, the composition of which depends on the amount of water and other conditions:
It is also capable of extracting water from other compounds, representing a strong dehydrating agent:
Phosphorus(V) oxide is widely used in organic synthesis. It reacts with amides, turning them into nitriles:
Carboxylic acids are converted to the corresponding anhydrides:
Phosphorus(V) oxide also interacts with alcohols, ethers, phenols and other organic compounds. In this case, the P-O-P bonds are broken and organophosphorus compounds are formed. Reacts with NH3 and hydrogen halides, forming ammonium phosphates and phosphorus oxyhalides:
When P4O10 fuses with basic oxides, it forms various solid phosphates, the nature of which depends on the reaction conditions.
Receipt
Phosphorus(V) oxide is produced by burning phosphorus. The technological process takes place in a combustion chamber and includes the oxidation of elemental P with pre-dried air, the precipitation of P4O10 and the purification of exhaust gases. The resulting pentoxide is purified by sublimation.
The technical product has the appearance of a white snow-like mass consisting of a mixture of different forms of P4O10.
Application
P4O10 is used as a desiccant for gases and liquids. It is also an intermediate product in the production of orthophosphoric acid H3PO4 by the thermal method.
Widely used in organic synthesis in dehydration and condensation reactions.
Phosphorus value
- phosphorus is included in nucleic acids, which take part in the processes of growth, cell division, storage and use of genetic information
- phosphorus is contained in the bones of the skeleton (about 85% of the total amount of phosphorus in the body)
- phosphorus is necessary for the normal structure of teeth and gums
- ensures proper functioning of the heart and kidneys
- phosphorus is involved in the processes of accumulation and release of energy in cells
- participates in the transmission of nerve impulses
- helps the metabolism of fats and starches.
The inorganic element phosphorus, P, is found in the human body in the form of phosphorus compounds - inorganic phosphates and lipids or nucleotides.
Previous10111213141516171819202122232425Next
Physical properties
Phosphorus P has several allotropic modifications: white, red, black.
Obtaining phosphorus P
Free phosphorus P obtained from natural calcium phosphate by heating it with sand ( SiO2) and coal in an electric oven at high temperature:
Chemical properties of phosphorus - P
White phosphorus more reactive than red.
Be careful - phosphine!
It oxidizes easily and spontaneously ignites in air.
When oxidized, white phosphorus glows in the dark, chemical energy is converted into light energy.

Phosphorus compounds P with metals are called phosphides. They are easily decomposed by water to form gas phosphine (PH3).
Phosphine - PH3
4. When there is a large excess of chlorine, phosphorus pentachloride is formed:

Phosphorus oxides and acids
Phosphorus forms with oxygen three oxides :
P2O3 – phosphorous anhydride – phosphorus oxide (III);
P2O5—phosphoric anhydride—phosphorus (V) oxide;
(P2O4 is phosphorus tetroxide).
P2O3 obtained by the slow oxidation of phosphorus (with a lack of oxygen):
When exposed to cold water, it forms phosphorous acid – H3PO3.
P2O5 is formed when phosphorus burns in air (with an excess of oxygen):
Acids
Phosphoric anhydride P2O5, depending on the temperature, can add different amounts of water, forming acids of different compositions:

What matters most is ortho phosphoric acid -H3PO4.
It can be obtained as follows:
1. Boiling metaphosphoric acid:
2. Oxidation of red phosphorus:
3. The action of sulfuric acid on calcium phosphate:
©2015 arhivinfo.ru All rights belong to the authors of the posted materials.
Chemistry tutor
Continuation. See in No. 22/2005;
3, 4, 7, 10, 11, 21/2007;
2, 7, 11, 18, 19, 21/2008;
1, 3, 10, 11/2009
1, 2, 3, 5, 6, 8, 9, 11, 13, 15, 16, 18, 22/2006;
LESSON 30 10th grade
(first year of study)
Phosphorus and its compounds
2. 1. Position in the table of D.I. Mendeleev, structure of the atom. Short story
discoveries and origin of the name.
3. Physical properties.
4. Chemical properties.
5. Being in nature.
6. Basic methods of obtaining
7. The most important phosphorus compounds. Phosphorus is found in main subgroup Group V periodic table D.I. Mendeleev. His 1electronic formula 2 2electronic formula 2 s 6 3electronic formula 2 s p 3, this R

-element. The characteristic oxidation states of phosphorus in compounds are –3, +3, +5; The most stable oxidation state is +5. In compounds, phosphorus can be part of both cations and anions, for example: Phosphorus gets its name from the ability of white phosphorus to glow in the dark. Greek word
translated as “carrying light.” Phosphorus owes this name to its discoverer, the alchemist Brand, who, mesmerized by the glow of white phosphorus, came to the conclusion that he had received the philosopher's stone.
Phosphorus can exist in the form of several allotropic modifications, the most stable of which are white, red and black phosphorus. Molecule white phosphorus
(the most active allotrope) has a molecular crystal lattice, at the nodes of which there are tetraatomic P 4 molecules of a tetrahedral structure.
White phosphorus is soft, like wax, melts and boils without decomposition, and has a garlicky odor. In air, white phosphorus quickly oxidizes (glows greenish), and self-ignition of fine white phosphorus is possible. Insoluble in water (stored under a layer of water), but soluble in organic solvents. Toxic (even in small doses, MPC = 0.03 mg/m3). Has very high chemical activity. When heated without air access to 250–300 ° C, it turns into red phosphorus.
Red phosphorus looks like graphite.
In structure, it is an inorganic polymer, the molecules of which have a layered structure.
Semiconductor. Not poisonous. Chemical activity is significantly lower than that of white phosphorus. Stable in air. When heated, it turns into red phosphorus.
Chemical properties
The most chemically active is white phosphorus (but in practice they prefer to work with red phosphorus). It can exhibit the properties of both an oxidizing agent and a reducing agent in reactions, for example:
4P + 3O 2 2P 2 O 3,
4P + 5O 2 2P 2 O 5.
Metals (+/–)*:
3Ca + 2P Ca 3 P 2,
3Na + P Na 3 P,
![]()
Cu + P reaction does not occur.
Non-metals (+):

2P + 3I 2PI 3,
6P + 5N 2 2P 2 N 5 .
Basic oxides (–).
Acidic oxides (–).
Alkalis (+):
Acids (not oxidizing agents) (–).
Oxidizing acids (+):
3P (red) + 5HNO 3 (dil.) + 2H 2 O = 3H 3 PO 4 + 5NO,
P (red) + 5HNO 3 (conc.) H 3 PO 4 + 5NO 2 + H 2 O,
2P (cr.) + H 2 SO 4 (conc.) 2H 3 PO 4 + 5SO 2 + 2H 2 O.
Salts (–)**.
In nature, phosphorus occurs in the form of compounds (salts), the most important of which are phosphorite (Ca 3 (PO 4) 2), chlorapatite (Ca 3 (PO 4) 2 CaCl 2) and fluorapatite (Ca 3 ( PO 4) 2 CaF 2).
Calcium phosphate is found in the bones of all vertebrates, causing their strength. Phosphorus is obtained in electric furnaces by fusing calcium phosphate, sand and coal without access to air: Ca 3 (PO 4) 2 + 3SiO 2 + 5C 2P + 5CO + 3CaSiO 3.
TO
the most important connections phosphorus include: phosphine, phosphorus(III) oxide, phosphorus(V) oxide, phosphoric acids. F o f i n
This hydrogen compound of phosphorus, a colorless gas with a garlicky fishy odor, is highly poisonous.
Poorly soluble in water, but highly soluble in organic solvents. Much less stable than ammonia, but is a stronger reducing agent.
Practical significance
does not have.
To obtain phosphine, a direct synthesis reaction from simple substances is usually not used;
The most common method for producing phosphine is the hydrolysis of phosphides:
Ca 3 P 2 + 6HOH = 3Ca(OH) 2 + 2PH 3.
In addition, phosphine can be obtained by a disproportionation reaction between phosphorus and alkali solutions:
4P + 3KOH + 3H 2 O PH 3 + KPO 2 H 2 , or from phosphonium salts:
PH 4 I PH 3 + HI,
PH 4 I + NaOH PH 3 + NaI + H 2 O.
2PH 3 + H 2 SO 4 (PH 4) 2 SO 4.
Redox properties.
Phosphine is a strong reducing agent:
2PH 3 + 4O 2 P 2 O 5 + 3H 2 O,
PH 3 + 8AgNO 3 + 4H 2 O = H 3 PO 4 + 8Ag + 8HNO 3.
O s i d p h o s p h o r a (III) Oxide P 2 O 3 (true formula – P 4 O 6) – white crystalline substance
, a typical acidic oxide. When reacting with water in the cold, it forms phosphorous acid (medium strength):

P 2 O 3 + 3H 2 O = 2H 3 PO 3
Since phosphorous acid is dibasic, when phosphorus trioxide reacts with alkalis, two types of salts are formed - hydrophosphites and dihydrophosphites.
For example:
P 2 O 3 + 4NaOH = 2Na 2 HPO 3 + H 2 O,
P 2 O 3 + 2NaOH + H 2 O = 2NaH 2 PO 3.
Phosphorus dioxide P 2 O 3 is oxidized by atmospheric oxygen to pentoxide:
P 2 O 3 + O 2 P 2 O 5 .
Phosphorus trioxide and phosphorous acid are fairly strong reducing agents.
Phosphorus(III) oxide is obtained by the slow oxidation of phosphorus in the absence of oxygen:
4P + 3O 2 2P 2 O 3 . Phosphorus(V) oxides and phosphoric acids Phosphorus pentoxide P 2 O 5 (true formula – P 4 O 10) is a white hygroscopic crystalline substance. In solid and
gaseous states:
the molecule exists in the form of a dimer and monomerizes at high temperatures. Typical acid oxide. It dissolves very well in water, forming a number of phosphoric acids:

metaphosphoric:
P 2 O 5 + H 2 O = 2HPO 3

pyrophosphoric (diphosphoric):
P 2 O 5 + 2H 2 O = H 4 P 2 O 7

orthophosphoric (phosphoric) P 2 O 5 + 3H 2 O = 2H 3 PO 4 Phosphorus pentoxide exhibits all the properties characteristic of
acid oxides
, For example:
P 2 O 5 + 3H 2 O = 2H 3 PO 4,

P 2 O 5 + 3CaO 2Ca 3 (PO 4) 2; can form three types of salts:
Oxidative properties
are not typical for him, because The oxidation state +5 is very stable for phosphorus. Phosphorus pentoxide is obtained by burning phosphorus in a sufficient amount of oxygen: 4P + 5O 2 2P 2 O 5 . Orthophosphoric acid H 3 PO 4 is a colorless crystalline substance, very soluble in water, hygroscopic. It is a triprotic acid of medium strength; does not have pronounced oxidizing properties.
Reveals everything
Chemical properties
, characteristic of acids, forms three types of salts (phosphates, hydrogen phosphates and dihydrogen phosphates):

2H 3 PO 4 + 3Ca = Ca 3 (PO 4) 2 + 3H 2,
H3PO4+Cu,
Ca 3 (PO 4) 2 + 3H 2 SO 4 = 2H 3 PO 4 + 3CaSO 4,
and also by the thermal method:
Ca 3 (PO 4) 2 + 3SiO 2 + 5C 3СaSiO 3 + 2P + 5CO,
4P + 5O 2 2P 2 O 5,
P 2 O 5 + 3H 2 O = 2H 3 PO 4.
Calcium phosphate is found in the bones of all vertebrates, causing their strength. laboratory methods the production of orthophosphoric acid refers to the effect of dilute nitric acid on phosphorus:
3P (cr.) + 5HNO 3 (dil.) + 2H 2 O = 3H 3 PO 4 + 5NO,
interaction of metaphosphoric acid with water when heated:
HPO 3 + H 2 O H 3 PO 4 .
In the human body, orthophosphoric acid is formed by the hydrolysis of adenosinotriphosphoric acid (ATP):
ATP ADP + H 3 PO 4 .
Qualitative reaction to phosphate ion is a reaction with a silver cation; A yellow precipitate is formed, insoluble in slightly acidic media:
3Ag + + = Ag 3 PO 4,
3AgNO3 + K3PO4 = Ag3PO4 + 3KNO3.
In addition to the above phosphoric acids (containing phosphorus in the +5 oxidation state), many other oxygen-containing acids are known for phosphorus. Here are some of the most important representatives.
Phosphorous(HPO 2 H 2) is a monobasic acid of medium strength. Its second name is phosphine:

Salts of this acid are called hypophosphites, or phosphites, for example KPO 2 H 2.
Phosphorous(H 3 PO 3) is a dibasic acid of medium strength, slightly weaker than hypophosphorous. It also has a second name – phosphonic:

Its salts are called phosphites, or phosphonates, for example K 2 PO 3 H.
Diphosphoric (pyrophosphoric)(H 4 P 2 O 7) - a tetrabasic acid of medium strength, slightly stronger than phosphoric acid:

Salts are diphosphates, for example K 4 P 2 O 7.
Test on the topic “Phosphorus and its compounds”
1. Eliminate the “extra” element from those listed according to the principle of the possibility of forming allotropic modifications:
a) oxygen; b) nitrogen;
c) phosphorus; d) sulfur.
2. When 42.6 g of phosphoric anhydride and 400 g of 15% sodium hydroxide solution react, the following is formed:
a) sodium phosphate;
b) sodium hydrogen phosphate;
c) a mixture of phosphate and sodium hydrogen phosphate;
d) a mixture of sodium hydro- and dihydrogen phosphate.
3. The sum of the coefficients in the equation electrolytic dissociation potassium phosphate is equal to:
a) 5; b) 3; at 4; d) 8.
4. Number of electrons in the outer level of a phosphorus atom:
a) 2; b) 3; at 5; d) 15.
5. Phosphorus obtained from 33 g of technical calcium phosphate was burned in oxygen.
The resulting phosphorus(V) oxide reacted with 200 ml of a 10% sodium hydroxide solution (density 1.2 g/ml) to form a medium salt. The mass of impurities in a technical sample of calcium phosphate (in g) is:
6. a) 3.5; b) 1.5; at 2; d) 4.8.
a) 2; b) 12; c) 14; d) 10.
7. The number of hydrogen atoms contained in 4.48 liters (n.s.) of phosphine is:
a) 1.2 10 23; b) 0.6 10 23;
c) 6.02 10 23; d) 3.6 10 23 .
8. At a temperature of 30 °C, a certain reaction takes place in 15 s, and at 0 °C - in 2 minutes.
Van't Hoff coefficient for this reaction:
9. a) 2.4; b) 2; c) 1.8; d) 3.
Phosphoric acid can react with the following substances:
a) copper(II) oxide; b) potassium hydroxide;
10. c) nitric acid; d) zinc.
The sum of the coefficients in the reaction between phosphorus and Berthollet salt is equal to:
a) 9; b) 6; c) 19; d) such a reaction is impossible.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Key to the test | b | V | b | b | Key to the test | A | Key to the test | G | b |
a, b, d
Tasks and exercises on phosphorus and its compounds
1. Chain of transformations:
2. Phosphorus -> phosphorus pentoxide -> orthophosphoric acid -> calcium phosphate ® phosphoric acid.
3. Calcium phosphate -> phosphorus -> calcium phosphide -> phosphine -> phosphorus pentoxide -> phosphoric acid -> calcium dihydrogen phosphate.
4. Calcium phosphate -> A -> B -> C -> D -> E -> calcium phosphate. All substances contain phosphorus, in the scheme there are three ORPs in a row.
5. Phosphorus -> phosphorus pentoxide -> calcium phosphate -> phosphorus -> phosphine -> phosphoric acid -> calcium dihydrogen phosphate. Calcium phosphide (+ hydrochloric acid solution) -> A (+ oxygen) -> B (+ sodium hydroxide
| , deficiency) -> C (+ sodium hydroxide, excess) -> D (+ calcium hydroxide) -> E. |
1. Level A
With complete combustion of 6.8 g of the substance, 14.2 g of phosphorus pentoxide and 5.4 g of water were obtained.
37 ml of a 32% sodium hydroxide solution (density 1.35 g/ml) was added to the resulting reaction products.
![]()
Establish the formula of the starting substance and determine the concentration of the resulting solution.
Solution
Reaction equation: (P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(P) = 0.2 mol, (H) = 0.6 mol.
(P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol. m(P) = 6.2 g,
m
(H) = 0.6 g.

= 6.8 g.
(P) : (H) = 0.2: 0.6 = 1: 3.
Therefore, the formula of the starting substance is PH 3, and the reaction equation is:

then phosphoric acid is formed:

(H 3 PO 4) = 2(P 2 O 5) = 0.2 mol.
Phosphoric acid can react with alkali as follows:
Let us determine the amount of substance NaOH according to the conditions of the problem:
(P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(H 3 PO 4) : (NaOH) = 0.2: 0.4 = 1: 2, Therefore, reaction 2 occurs.(Na 2 HPO 4) = (H 3 PO 4) = 0.2 mol;
(P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(Na 2 HPO 4) = (P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol. M (Na 2 HPO 4) (Na 2 HPO 4) = 142 0.2 = 28.4 g;(r-ra) = (P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(P 2 O 5) +
m (Na 2 HPO 4) (Na 2 HPO 4) = 142 0.2 = 28.4 g;(H 2 O) + (P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(NaOH solution) = 14.2 + 5.4 + 37 1.35 = 69.55 g.
(Na 2 HPO 4) =(Na2HPO4)/
2. With complete electrolysis of 1 kg of iron(II) sulfate solution, 56 g of metal was released at the cathode. What mass of phosphorus can react with the substance released at the anode, and what will be the composition of the salt if the resulting reaction product is dissolved in 87.24 ml of a 28% sodium hydroxide solution (solution density 1.31 g/ml)?
(Na 2 HPO 4) = 12.4 g phosphorus; sodium hydrogen phosphate.
3. 20 g of a mixture consisting of barium sulfate, calcium phosphate, calcium carbonate and sodium phosphate were dissolved in water. The mass of the insoluble part was 18 g. When hydrochloric acid acted on it, 2.24 liters of gas (n.s.) were released and the mass of the insoluble residue was 3 g. Determine the composition of the initial mixture of salts by mass.
(Na 2 HPO 4) = Na 3 PO 4 – 2 g; BaCO 3 – 3 g;
CaCO 3 – 10 g; Ca 3 (PO 4) 3 – 5 g.
4. How many kg of phosphorus can be obtained from 1 ton of phosphorite containing 40% impurities? What is the volume at standard conditions? will the phosphine obtained from this phosphorus take?
(Na 2 HPO 4) = 120 kg P; 86.7 m 3 PH 3 .
5. 40 g of a mineral containing 77.5% calcium phosphate was mixed with excess sand and coal and heated without air in an electric furnace. Received simple substance dissolved in 140 g of 90% nitric acid. Determine the mass of sodium hydroxide that will be required to completely neutralize the oxidation product of a simple substance.
(Na 2 HPO 4) = 24 g NaOH.
| Level B |
1. To completely neutralize the solution obtained by hydrolysis of 1.23 g of some phosphorus halide, 35 ml of a 2M potassium hydroxide solution was required. Determine the formula of the halide.
(Na 2 HPO 4) = Phosphorus trifluoride.
2. A sample of anhydrous ethanol containing 0.5% phosphorus(V) oxide as an impurity was burned in a sufficient amount of oxygen.
(Na 2 HPO 4) = The resulting gases were separated, and the resulting solution was heated until gas evolution stopped, after which an equal weight 0.5% solution of potassium hydroxide was added to it. Determine the mass fractions of substances in the resulting solution.
K 2 HPO 4 – 0.261%;
3. KH 2 PO 4 – 0.204%.
(Na 2 HPO 4) = To 2 g of a mixture of hydrogen phosphate and potassium dihydrogen phosphate, in which the mass fraction of phosphorus is 20%, was added 20 g of a 2% solution of phosphoric acid. Calculate the mass fractions of substances in the resulting solution.
KH 2 PO 4 – 9.03%;
4. K 2 HPO 4 (remaining) – 1.87%.
(Na 2 HPO 4) = When a mixture of alkali metal hydride and phosphide with equal mass fractions was treated with water, a gas mixture with a nitrogen density of 0.2926 was formed. Determine what metal was included in the compounds.
5. 50 g of a mixture of calcium phosphate and calcium and ammonium carbonates was calcined, resulting in 25.2 g of a solid residue, to which water was added, and then excess carbon dioxide was passed through. The mass of the undissolved residue was 14 g. Determine the mass of ammonium carbonate in the original mixture.
With complete combustion of 6.8 g of the substance, 14.2 g of phosphorus pentoxide and 5.4 g of water were obtained.
When the mixture is calcined, the following processes occur:
1) Ca 3 (PO 4) 2;
2) ![]()
3) (NH 4) 2 CO 3 2NH 3 + CO 2 + H 2 O.
The solid residue contains Ca 3 (PO 4) 2 and CaO.
After adding water:
4) Ca 3 (PO 4) 2 + H 2 O;
5) CaO + H 2 O = Ca(OH) 2.
After passing carbon dioxide:
6) Ca(OH) 2 + H 2 O + CO 2 = Ca(HCO 3) 2.
The undissolved residue is Ca 3 (PO 4) 2, therefore, (P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(Ca 3 (PO 4) 2) = 14 g.
Finding the mass of CaO:
(P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(CaO) = 25.2 – 14 = 11.2 g.
(CaO) = 11.2/56 = 0.2 mol,
(CaCO 3) = (CaO) = 0.2 mol,
(P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(CaCO 3) = 0.2 100 = 20 g.
(P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(NH 4) 2 CO 3 = (P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(mixtures) – (P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(Ca 3 (PO 4) 2) – (P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(CaCO 3) = 50 – 14 – 20 = 16 g.
Answer. (P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.(NH 4) 2 CO 3 = 16 g.
QUALITATIVE TASKS
1. Solid, white, highly soluble in water, compound A is an acid. When oxide B is added to aqueous solution A, a white, water-insoluble compound C is formed. As a result of calcination of substance C at high temperature in the presence of sand and coal, a simple substance is formed, which is part of A. Identify the substances, write the reaction equations.
Answer. Substances: A – H 2 PO 4, B – CaO,
C – Ca 3 (PO 4) 2.
2. A mixture of two solids, red (A) and white (B), ignites with slight friction. The reaction produces two white solids, one of which (C) dissolves in water to form an acidic solution. If calcium oxide is added to substance C, a white, water-insoluble compound is formed. Identify substances, write reaction equations.
Answer. Substances: A – P (cr.), B – KClO 3,
C – P 2 O 5 .
3. The water-insoluble white compound A, as a result of calcination at high temperatures with coal and sand in the absence of oxygen, forms a simple substance B, which exists in several allotropic modifications. When substance B is burned, compound C is formed, which dissolves in water to form acid E, which is capable of forming three types of salts. Identify substances, write reaction equations.
Answer. Substances: A – Ca 3 (PO 4) 2, B – P,
C – P 2 O 5, E – H 3 PO 4.
* The +/– sign means that this reaction does not occur with all reagents or under specific conditions.
** An interesting one is the oxidation-reduction reaction (ORR) that occurs when matches are lit:
To be continued
Phosphine formula………………………………………………………….....PH 3
Molecular weight………………………………………………………34.04
Color and appearance................................................... .......Colorless gas.
Melting point................................... - 133.5 °C.
Boiling temperature................................................ .... -87.7°C.
Evaporation pressure........................40 mm Hg. Art. at - 129.4°C.
Solubility in water........................26% by volume at 17°C.
Density........................1.18 (0°C, 760 mmHg) (Air-1).
Flash point................................................... .....100°C.
Lower explosive limit........... 1.79-1.89% of volume;
Odor appears when................................................... ......1.3 - 2.6 ppm.
At relatively high concentrations, phosphine is explosive.
Lower flammability limit (LCFL) – 1.79-1.89%
by volume or ……………………………..26.15-27.60 g/m 3, or 17000-18900 ml/m 3.
The latent heat of evaporation of phosphine is …………………………………102.6 cal/g.
Solubility in water is 0.52 g/l at a temperature of 20 0 C and a pressure of 34.2 kgf/cm 2.
Phosphine
– a highly toxic, colorless gas that is 1.5 times heavier than air, therefore, when used, it easily penetrates into all cracks and hard-to-reach places in the premises and effectively destroys eggs, larvae, pupae and adult insects.
It dissolves poorly in water and does not react with it. Soluble in benzene, diethyl ether, carbon disulfide. Phosphine is highly toxic, affects the nervous system, and disrupts metabolism. MPC = 0.1 mg/m³. The odor is noticeable at a concentration of 2-4 mg/m³; prolonged inhalation at a concentration of 10 mg/m³ is fatal.
Application of phosphine. When carrying out fumigation with phosphine, inorganic preparations based on aluminum and magnesium phosphides are used. The objects and technology for using preparations based on magnesium phosphide are identical to those based on aluminum phosphide. Admission of people and loading of warehouses is permitted after complete ventilation and if the phosphine content in the air of the working area is not higher than the maximum permissible concentration (0.1 mg/m³). Products are sold with a phosphine residue not higher than the MRL (0.1 mg/kg for grain, 0.01 mg/kg for grain processing products).
Phosphine gas is a strong poison for humans and other warm-blooded animals. Acute phosphine poisoning occurs at a concentration in the air of 568 mg/m3. Phosphine gas is highly toxic to insects that pest grain stocks. When working with it, it is advisable to have an understanding of method and mechanism of action on harmful organisms. Extremely permissible concentration(MPC) of phosphine in the air of the working area is 0.1 mg/m3. However, the smell of gas begins to be felt at lower concentrations (about 0.03 mg/m3). The maximum permissible level (ML) of phosphine in grain is 0.01 mg/kg; phosphine residues are not allowed in grain products. Grain and products of its processing can be used for food purposes only if the residual amounts of phosphine in them do not exceed the MRL.
Phosphine gas It is weakly sorbed by grain and grain products, therefore it is easily degassed. At the consumption rates recommended for disinsection, it does not change the quality of the grain and does not impair its seed properties. It was first used in 1934 for the fumigation of grain products. Currently, due to the ban on the use methyl bromide For fumigation purposes, phosphine is the main fumigant used to control harmful insects.