Malleable metal of silver-white color with high chemical reactivity: Iron corrodes quickly when exposed to high temperatures or high humidity. IN pure oxygen iron burns, and in a finely dispersed state it spontaneously ignites in air. Denoted by the symbol Fe (Latin Ferrum). One of the most common in earth's crust metals (second place after ).
See also:
STRUCTURE

Several polymorphic modifications have been established for iron, of which the high-temperature modification - γ-Fe (above 906°) forms a lattice of a face-centered cube of the Cu type (a 0 = 3.63), and the low-temperature modification - the α-Fe lattice of a centered cube of the α-Fe type ( a 0 = 2.86).
Depending on the heating temperature, iron can be found in three modifications, characterized by different crystal lattice structures:
- In the temperature range from the lowest to 910 ° C - a-ferrite (alpha ferrite), having a crystal lattice structure in the form of a centered cube;
- In the temperature range from 910 to 1390°C - austenite, the crystal lattice of which has the structure of a face-centered cube;
- In the temperature range from 1390 to 1535°C (melting point) - d-ferrite (delta ferrite). The crystal lattice of d-ferrite is the same as that of a-ferrite. The difference between them is only in other (larger for d-ferrite) distances between the atoms.
When liquid iron is cooled, primary crystals (crystallization centers) appear simultaneously at many points in the cooled volume. With subsequent cooling, new crystalline cells are built around each center until the entire supply of liquid metal is exhausted.
The result is a granular structure of the metal. Each grain has a crystal lattice with a certain direction of its axes.
With subsequent cooling of solid iron, during the transitions of d-ferrite to austenite and austenite to a-ferrite, new crystallization centers may appear with a corresponding change in grain size
PROPERTIES

In its pure form normal conditions it's a solid. It has a silver-gray color and a pronounced metallic luster. Mechanical properties iron include the level of hardness on the Mohs scale. It is equal to four (average). Iron has good electrical and thermal conductivity. The last feature can be felt by touching an iron object in a cold room. Because this material conducts heat quickly, it removes most of it from your skin in a short period of time, which is why you feel cold.
If you touch, for example, wood, you will notice that its thermal conductivity is much lower. Physical properties iron - these are its melting and boiling points. The first is 1539 degrees Celsius, the second is 2860 degrees Celsius. It can be concluded that characteristic properties iron - good ductility and fusibility. But that's not all. Also, the physical properties of iron include its ferromagnetism. What it is? Iron, magnetic properties which we can observe in practical examples every day is the only metal with such a unique distinctive feature. This is explained by the fact that this material is capable of magnetization under the influence of a magnetic field. And after the end of the action of the latter, the iron, the magnetic properties of which have just been formed, remains a magnet for a long time. This phenomenon can be explained by the fact that in the structure of this metal there are many free electrons that are able to move.
RESERVES AND PRODUCTION
Iron is one of the most common elements in solar system, especially on the terrestrial planets, in particular on Earth. A significant part of the iron of the terrestrial planets is located in the cores of the planets, where its content is estimated to be about 90%. The iron content in the earth's crust is 5%, and in the mantle about 12%.

Iron is quite widespread in the earth's crust - it accounts for about 4.1% of the mass of the earth's crust (4th place among all elements, 2nd among metals). In the mantle and crust, iron is concentrated mainly in silicates, while its content is significant in basic and ultrabasic rocks, and low in acidic and intermediate rocks.
Known big number ores and minerals containing iron. Greatest practical significance have red iron ore (hematite, Fe2O3; contains up to 70% Fe), magnetic iron ore (magnetite, FeFe 2 O 4, Fe 3 O 4; contains 72.4% Fe), brown iron ore or limonite (goethite and hydrogoethite, respectively FeOOH and FeOOH nH 2 O). Goethite and hydrogoethite are most often found in weathering crusts, forming so-called “iron hats”, the thickness of which reaches several hundred meters. They may also be of sedimentary origin, falling out of colloidal solutions in lakes or coastal areas of seas. In this case, oolitic, or legume, iron ores are formed. Vivianite Fe 3 (PO 4) 2 8H 2 O is often found in them, forming black elongated crystals and radial aggregates.
The iron content in sea water is 1·10−5 -1·10−8%
In industry, iron is obtained from iron ore, mainly from hematite (Fe 2 O 3) and magnetite (FeO Fe 2 O 3).
Exist various ways extraction of iron from ores. The most common is the domain process.
The first stage of production is the reduction of iron with carbon in a blast furnace at a temperature of 2000 °C. In a blast furnace, carbon in the form of coke, iron ore in the form of agglomerate or pellets, and flux (such as limestone) are fed from above, and are met by a stream of forced hot air from below.
In addition to the domain process, a common process is direct receipt gland. In this case, pre-crushed ore is mixed with special clay, forming pellets. The pellets are fired and treated in a shaft furnace with hot methane conversion products, which contain hydrogen. Hydrogen easily reduces iron without contaminating the iron with impurities such as sulfur and phosphorus, which are common impurities in coal. Iron is obtained in solid form and is subsequently melted in electric furnaces. Chemically pure iron is obtained by electrolysis of solutions of its salts.
ORIGIN

Origin telluric (terrestrial) iron is rarely found in basalt lavas (Uifak, Disko Island, off the western coast of Greenland, near Kassel, Germany). At both points, pyrrhotite (Fe 1-x S) and cohenite (Fe 3 C) are associated with it, which is explained by both the reduction by carbon (including from the host rocks) and the decomposition of carbonyl complexes such as Fe(CO) n. In microscopic grains, it has more than once been established in altered (serpentinized) ultrabasic rocks, also in paragenesis with pyrrhotite, sometimes with magnetite, due to which it arises when recovery reactions. Very rarely found in the oxidation zone of ore deposits, during the formation of swamp ores. Findings have been recorded in sedimentary rocks associated with the reduction of iron compounds with hydrogen and hydrocarbons.
Almost pure iron was found in lunar soil, which is associated with both meteorite falls and magmatic processes. Finally, two classes of meteorites - stony-iron and iron - contain natural iron alloys as a rock-forming component.
APPLICATION

Iron is one of the most used metals, accounting for up to 95% of global metallurgical production.
Iron is the main component of steels and cast irons - the most important structural materials.
Iron can be part of alloys based on other metals - for example, nickel.
Magnetic iron oxide (magnetite) is an important material in the production of long-term computer memory devices: hard drives, floppy disks, etc.
Ultrafine magnetite powder is used in many black and white laser printers mixed with polymer granules as a toner. This uses both the black color of the magnetite and its ability to stick to the magnetized transfer roller.
The unique ferromagnetic properties of a number of iron-based alloys contribute to their widespread use in electrical engineering for magnetic cores of transformers and electric motors.
Iron(III) chloride (ferric chloride) is used in amateur radio practice for etching printed circuit boards.
Ferrous sulfate heptate (ferrous sulfate) mixed with copper sulfate is used to combat harmful fungi in gardening and construction.
Iron is used as an anode in iron-nickel batteries and iron-air batteries.
Aqueous solutions of ferrous and ferric chlorides, as well as its sulfates, are used as coagulants in the purification processes of natural and Wastewater in water treatment of industrial enterprises.
Iron - Fe
CLASSIFICATION
Hey's CIM Ref1.57
| Strunz (8th edition) | 1/A.07-10 |
| Nickel-Strunz (10th edition) | 1.AE.05 |
| Dana (7th edition) | 1.1.17.1 |
Lesson objectives:
- Introduce students to the side group element Periodic table– iron, its structure, properties.
- Know the location of iron in nature, methods of obtaining it, application, physical properties.
- Be able to characterize iron as an element of a secondary subgroup.
- Be able to prove the chemical properties of iron and its compounds, write reaction equations in molecular, ionic, redox form.
- To develop students’ skills in composing equations of reactions involving iron, to form students’ knowledge of qualitative reactions to iron ions.
- Cultivate interest in the subject.
Equipment: iron (powder, pin, plate), sulfur, oxygen flask, hydrochloric acid, iron(II) sulfate, iron(III) chloride, sodium hydroxide, red and yellow blood salts.
DURING THE CLASSES
I. Organizational moment
II. Checking homework
III. Learning new material
1. Teacher's introduction.
– The importance of iron in life, its role in the history of civilization. One of the most common metals in the earth's crust is iron. It began to be used much later than other metals (copper, gold, zinc, lead, tin), which is most likely due to the low similarity of iron ore with the metal. To primitive people It was very difficult to guess that metal could be obtained from ore, which could be successfully used in the manufacture of various objects; the lack of tools and necessary devices for organizing such a process affected it. Quite a long time passed before man learned to extract iron from ore and make steel and cast iron from it. long time.
At the moment, iron ores are a necessary raw material for ferrous metallurgy, those minerals that no developed industrial country can do without. Annual world production of iron ore is approximately 350,000,000 tons. They are used for smelting iron (carbon content 0.2-0.4%), cast iron (2.5-4% carbon), steel (2.5-1.5% carbon). Steel has the most widespread use in industry than iron and cast iron, which is why there is greater demand for its smelting.
To smelt cast iron from iron ores, blast furnaces are used that run on coal or coke; steel and iron are melted from cast iron in reverberatory open-hearth furnaces, Bessemer converters, or the Thomas method.
Ferrous metals and their alloys are of great importance in the life and development of human society. All kinds of household and consumer items are made of iron. For the construction of ships, aircraft, railways, cars, bridges, railways, various buildings, equipment and other things, hundreds of millions of tons of steel and cast iron are used. There is no such industry Agriculture and industry in which iron and its various alloys would not be used.
The few minerals commonly found in nature that contain iron are iron ore. Such minerals include: brown iron ore, hematite, magnetite, and others that form large deposits and occupying huge areas.
The chemical relation of magnetite or magnetic iron ore, which has an iron-black color and a unique property - magnetism, is a compound consisting of iron oxide and iron oxide. In the natural environment it can be found both in the form of granular or solid masses, and in the form of well-formed crystals. Iron ore is richest in the metallic iron content of magnetite (up to 72%).
The largest deposits of magnetite ores in our country are located in the Urals, in the Vysokaya, Blagodat, Magnitnaya mountains, in some areas of Siberia - the Angara River basin, Mountain Shoria, on the territory of the Kola Peninsula.
2. Work with the class. Characteristics of iron as a chemical element
a) Position in the periodic table:
Exercise 1. Determine the position of iron in the Periodic Table?
Answer: Iron is located in the 4th major period, even row, 8th group, minor group.
b) structure of the atom:
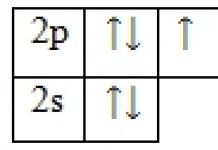
Task 2. Draw the composition and structure of the iron atom, electronic formula and cells.
Answer: Fe +3 2) 8) 14) 2)metal
p = 26
e = 26
n = (56 – 26) = 301s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2
Question. On which layers of iron are the valence electrons located? Why?
Answer. Valence electrons are located on the last and penultimate layers, since this is an element of the secondary subgroup.
Iron is classified as a d-element; it is part of the triad of elements - metals (Fe-Co-Ni);
c) redox properties of iron:
Question. What is iron - an oxidizing agent or a reducing agent? What oxidation states and valence does it exhibit?
Answer:
Fe 0 – 2e = Fe +3) reducing agent
Fe 0 – 3e = Fe +3
s.o.+ 2,+ 3; valency = II and III, valency 7 – does not show;
d) iron compounds:
FeO – basic oxide
Fe(OH) 2 – insoluble base
Fe 2 O 3 – oxide with signs of amphotericity
Fe(OH) 3 – a base with signs of amphotericity
Volatile hydrogen compounds- No.
d) being in nature.
Iron is the second most abundant metal in nature (after aluminum). In its free state, iron is found only in meteorites. The most important natural compounds:
FeO*3HO – brown iron ore,
FeO – red iron ore,
FeO (FeO*FeO) – magnetic iron ore,
FeS – iron pyrite (pyrite)
Iron compounds are found in living organisms.
3. Characteristics simple substance gland
a) molecular structure, type of bond, type of crystal lattice; (independent)
b) physical properties of iron
Iron is a silver-gray metal that has great malleability, ductility and strong magnetic properties. The density of iron is 7.87 g/cm 3, the melting point is 1539 t o C.
c) chemical properties of iron:
Iron atoms donate electrons in reactions and exhibit oxidation states of + 2, + 3 and sometimes + 6.
In reactions, iron is a reducing agent. However, at ordinary temperatures it does not interact even with the most active oxidizing agents (halogens, oxygen, sulfur), but when heated it becomes active and reacts with them:
2Fe +3Cl 2 = 2FeCl 3 Iron(III) chloride
3Fe + 2O 2 = Fe 2 O 3 (FeO*Fe O) Iron(III) oxide
Fe +S = FeS Iron(II) sulfide
At very high temperature iron reacts with carbon, silicon and phosphorus.
3Fe + C = Fe 3 C Iron carbide (cementite)
3Fe + Si = Fe 3 Si Iron silicide
3Fe + 2P = Fe 3 P 2 Iron phosphide
Iron reacts with complex substances.
In humid air, iron quickly acidifies (corrodes):
4Fe + 3O 2 + 6H 2 O = 4Fe(OH) 3
Fe(OH) 3 ––> FeOOH + H 2 O
Rust
Iron is in the middle of the electrochemical voltage series of metals, therefore it is a metal average activity. The reducing ability of iron is less than that of alkali, alkaline earth metals and aluminum. Only at high temperatures does hot iron react with water:
3Fe + 4H 2 O = Fe 3 O 4 + 4H 2
Iron reacts with dilute sulfuric and hydrochloric acids, displacing hydrogen from them:
Fe + 2HCl = FeCl 2 + H 2
Fe + H 2 SO 4 = FeSO 4 + H 2
Fe 0 + 2H + = Fe 2+ + H 2 0
At ordinary temperatures, iron does not interact with concentrated sulfuric acid, since it is passivated by it. When heated, concentrated sulfuric acid oxidizes iron to iron(III) sulfate:
2Fe + 6H 2 SO 4 = Fe 2 (SO 4) 3 + 3SO 2 + 6H 2 O
Diluted Nitric acid oxidizes iron to iron(III) nitrate:
Fe + 4HNO 3 = Fe(NO 3) 3 + NO + 2H 2 O
Concentrated nitric acid passivates iron.
From salt solutions, iron displaces metals that are located to the right of it in the electrochemical voltage series:
Fe + CuSO 4 = FeSO 4 + Cu,
d) use of iron (on your own)
e) receiving (together with students)
In industry, iron is obtained by reducing it from iron ores with carbon (coke) and carbon monoxide (II) in blast furnaces.
The chemistry of the blast furnace process is as follows:
C + O = CO
CO + C = 2CO
3Fe 2 O 3 + CO = 2Fe 3 O 4 + CO 2
Fe 3 O 4 + CO = 3FeO + CO 2
FeO + CO = Fe + CO 2
4. Iron compounds
Chemical properties connection data.

Addition. Iron(II) compounds are unstable, they can oxidize and turn into iron(III) compounds
Fe +2 Cl 2 + Cl 2 = Fe +3 Cl 3 make up redox house
Fe +2 (OH) + H 2 O + O 2 = Fe +3 (OH) 3 schemes, equalize.

Chemical properties of these compounds

Also, a qualitative reaction to Fe +2 is the reaction of iron(II) salts with a substance called red blood salt K3 - this is a complex compound.
3FeCl + 2K 3 = Fe 3)