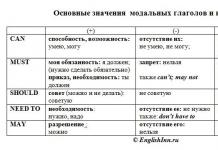
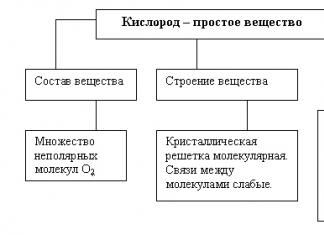
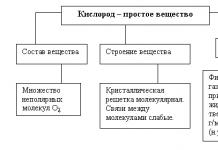
Chemical reactions are processes accompanied by a change in the distribution of electrons in the outer orbitals of the atoms of the reacting substances. The driving force of chemical reactions is the desire to form new compounds that have less free energy and, therefore, are more stable.
Substances that enter into a chemical reaction are called starting substances (compounds) or reagents. One of the reagents is usually called a substrate. This is, as a rule, the substance in which the old bond at the carbon atom is broken and a new bond is formed. The compound acting on the substrate is called an attacking reagent or reaction particle.
For example, when chlorinating alkanes:
CH 3 – CH 3 + C1 2 ® CH 3 – CH 2 C1 + HC1
ethane chloroethane chloride hydrogen chloride
ethane is the substrate and chlorine is the reactant.
During a chemical transformation, usually not the entire molecule changes, but only part of it - the reaction center.
A reaction center is an atom or group of atoms directly involved in a given reaction. chemical reaction.
Thus, when an organic base, methylamine, interacts with hydrochloric acid, methylamine is the substrate, and hydrochloric acid is the reagent. The reaction center is the nitrogen atom of the amino group. It is the lone electron pair of nitrogen that is directly attacked by the proton and attaches it.
CH 3 – N H 2 + H + C1 – ® CH 3 – N H 3 + C1 –
methylamine hydrogen chloride methyl ammonium chloride
Compounds formed during chemical interaction, are called reaction products.
Majority organic reactions includes several successive (elementary) stages. A detailed description of the totality and sequence of these stages is called a mechanism. A reaction mechanism is often a hypothesis proposed at a given level of scientific development to explain experimental data. It can be refined and even changed with the emergence of new experimental facts and the deepening of theoretical concepts.
Establishing the mechanism of organic reactions is quite difficult task. To solve it it is necessary to modern level knowledge to have a complete understanding of the intermediate stages and intermediate substances (intermediates), the nature of the interaction of reacting particles, the nature of the rupture and formation of bonds, energy changes chemical system along the entire path of its transition from the initial state to the final state. The mechanism must be consistent (be adequate) with the stereochemistry and kinetics of the process.
The overall rate of a complex chemical reaction is determined (limited) by the rate of its slowest stage, and the rate of the constituent elementary reactions is determined by their activation energy E a. Activation energy is the minimum additional amount of energy compared to the average required to carry out an effective collision of molecules leading to interaction. It can also be defined as the energy required for the system to achieve transition state, otherwise called an activated complex, the transformation of which into reaction products occurs spontaneously. The lower the activation energy of a reaction, the higher its speed. (This situation was discussed in more detail in the first part of the manual).
In the case of multistage processes, some stages include the formation of intermediates - unstable intermediate particles. Organic ions or radicals often act as intermediates. Their relative stability and, therefore, the probability of formation increases with increasing possibility of charge distribution (delocalization) or the appearance of an unpaired electron in a given particle.
To reduce the activation energy and, accordingly, increase the rate of a chemical reaction, catalysts are used. A catalyst is a chemical substance that speeds up a reaction, but is not part of the final reaction products. Theoretically, the amount of catalyst, unlike other reagents, does not change after the reaction. The principle of operation of a catalyst is to reduce the activation energy of a reaction. The catalyst reacts with the starting material to form an intermediate having a lower activation energy. The resulting intermediate is exposed to a reagent and then cleaved into a product and a catalyst. The catalyst then reacts with the starting material again, and this catalytic cycle is repeated many times. The catalyst does not affect the equilibrium position between the initial and final products, but reduces the time to reach the equilibrium position.
Substances that reduce the rate of a reaction are called inhibitors.
Studying the mechanisms of chemical reactions helps solve the following problems:
– systematize experimental data (knowledge of the reaction mechanism makes it possible to detect similarities and differences between reactions);
– optimize synthesis conditions (knowledge of the reaction mechanism allows you to determine the best conditions for obtaining the required product with the best yield at the lowest cost);
– predict reactivity (having established the reaction mechanism for one of the homologues, one can confidently predict the direction of the reaction for other members homologous series);
– allows you to carry out math modeling processes;
– provides intellectual satisfaction to the researcher.
1. Explain the difference between the concepts of “substrate” and “attack reagent”.
2. Define the activation energy of a reaction.
3. How does the introduction of a catalyst affect the activation energy of the reaction?
4. In the presence of oxygen, the rate of methane chlorination slows down. In this case, can oxygen be called a catalyst or an inhibitor of the reaction?
5. What particles can act as intermediates?
This difficult-to-understand article examines the psyche from the perspective of systematic approach. Much attention is paid to the emotional sphere. In particular, the systemic energetic concept of emotions is described.
Supporting articles:
In the most general view the psyche can be represented as an open functional system consisting of three elements:
- processes of mental image formation: attention, sensation, perception, emotions, thinking, memory
- reasons that stimulate mental activity: needs, motives
- purposeful mental activity: activity
The essence of the functioning of such a system in a simplified form is that the satisfaction of certain needs becomes a motive-goal, which activates the processes of formation of a mental image, and the mental image, in turn, activates activities aimed at satisfying the need and motive-goal that caused this activity . It should be noted that since all these elements are connected in a feedback system, where the usual cause-and-effect relationships do not operate, then, strictly speaking, it does not matter which element to start considering such a system with. However, according to the established everyday and scientific tradition, to facilitate understanding of the functioning of such a system, it is customary to begin with an analysis of the motivating reasons - needs, goals and motives, then move on to consideration of the formation of a mental image and, finally, to consideration of activity, which, on the one hand, is a consequence mental activity, and on the other hand, aimed at stopping this activity.
By applying the general scheme of the control system to the psyche, we can fill it with specific psychological content:
- management goals are to satisfy needs and motives, the study of which belongs to the subject of psychology (the traditional subject of psychoanalysis);
- means is a mental image, the study of which also relates to the subject of psychology (the traditional subject of Gestalt psychology);
- the result is an activity aimed at achieving a goal, the study of which undoubtedly belongs to the subject of psychology (the traditional subject of behaviorism and, by the way, the domestic theory of activity).
Thus, the subject of study of psychology is self-management of the purposeful vital activity of the body.
Define anyone scientific concept- means to explain it with the help of other, already known concepts, to indicate its place among other, already known phenomena, while highlighting the specific features inherent only to this concept. Let us apply this rule to determine the subject of psychology.
The psyche is inherent in living organisms and is absent in inanimate objects - physical bodies. Much has been written about how living matter differs from nonliving matter. scientific works, which agree that living organisms are capable of purposeful life activity. Inanimate, inanimate objects do not have this ability. Currently, no one argues with the position that active, purposeful life activity is possible only if there is the possibility of self-management of this life activity.
The key concept in this definition of the subject of psychology is the concept of “management”.
It is unlikely that anyone will argue that the world is infinite and completely unknowable and that some unknowable entity is possible, if you like, God or Nature, which influences and perhaps controls the soul of a living organism, while the soul that allows it cognition controls the organism itself. The soul – “psyche”, psyche – controls a person as an integral object, without singling out its individual organs or component parts. Various sciences that study man, defining their subject of study, highlight various aspects of the life of the body, and the aspect of managing the active, purposeful life of the body has become the subject of study of a science whose name includes the word “soul” - the science of psychology. In monographs devoted to theoretical problems of control in technology, cybernetics, psychology, etc. The control mechanism has long been understood as a feedback system, which includes in its structure three main components connected by both direct and feedback.
Let us note that this model in no way pretends to demonstrate the complexity and versatility of the control mechanism. This model emphasizes the systemic nature of management functioning, which involves direct and feedback connections of individual elements.
In Russian psychology, attempts have also been made repeatedly to present the structure and functioning of the psyche as a feedback system. This is a reflex ring, and N.A. Bernstein’s systematic approach to the analysis of movements, and P.K. Anokhin’s action acceptor and other more or less well-known attempts to explain the functioning of the psyche using systemic principles. Considering the psyche as a functional system, these authors certainly strived for holistic understanding of the psyche when all elements of the psyche are logically connected with each other.
The idea of the integrity of the psyche and the systemic interconnection of individual components of the psyche - motive, image and action - is most clearly reflected in the works of M.G. Yaroshevsky, who used a systematic approach for a categorical analysis of “... the development of psychological cognition as an activity.” He convincingly showed that the weakness and one-sidedness of popular psychological theories - psychoanalysis, Gestalt psychology and behaviorism lies precisely in the fact that these theories do not use a comprehensive, holistic, systematic approach to the study of the psyche and, ultimately, are limited in understanding the subject of psychology. Thus, psychoanalysis, Gestalt psychology and behaviorism analyze, respectively, needs and motives, mental image and activity, but do not consider the psyche as a whole, in the fullness of its properties.
It should be noted that the systemic concept of the psyche is broader than any traditional psychological theory, including the theory of activity, and at the same time does not contradict any of them. It is in it that a holistic approach to the psyche is realized.
So, in accordance with the systemic concept of the psyche:
– psyche – open system self-government of purposeful life activity inherent in a living organism;
– the psyche, understood as a system of self-government of the purposeful vital activity of the organism, has its own internal logic and can be considered both from the point of view of the functioning of individual elements of the system and their interrelation, and from the point of view of the functioning of the system as a whole.
In the light of the systems approach, the psyche is a multi-level, self-organizing, dynamic and open system, which differs in a number of ways: specific properties and characteristics.
1. The active and selective nature of reflecting the phenomena of reality, their relationships and interconnections, allowing the subject not only to navigate the world around him, but also to cognize it. This system property is manifested in two fundamental characteristics:
a) sensitivity – general ability to sense;
b) non-intentionality, uncharacteristic of physical objects, that is, a focus on an external other, which at higher levels of mental development becomes arbitrary.
2. “Advanced” nature of mental reflection, which also manifests itself in two main abilities:
a) anticipation or the ability to foresight, not only ascertaining, but also the anticipatory / predictive nature of information processes;
b) the ability to build a time perspective, plan and organize activities over time.
3. The ability to transform the energy of external influences into holistic information about the world (picture of the world), which at the highest levels of mental development can be realized, analyzed and comprehended.
4. Active and purposeful nature of adaptation (adaptation) to the surrounding world.
5. Conditionality of development and self-organization processes by factors of the sociocultural environment. This systemic property is expressed in the mediation of higher mental functions by experience social interaction and sign systems.
6. At higher levels of development, there is a tendency towards such complex forms of organization and self-regulation as consciousness, self-awareness, personality, which presupposes:
a) active reflection not only outside world(reflex activity), but also one’s own internal states and processes (reflection);
b) self-determination i.e. active goal setting and self-determination.
7. Axiological (value) and meaning-forming nature of the highest forms of organization of the psyche:
a) regulation of mental activity through values, meanings and value orientations;
b) the ability to realize the basic values of culture and creative meaning-making.
Psychic phenomena
The psyche manifests itself in mental phenomena.
All mental phenomena are divided into three groups:
- mental processes
- mental states
- mental properties of personality.
Mental processes is a dynamic reflection of reality in various forms psychic phenomena. They are divided into cognitive (these include sensations and perceptions, ideas and memory, thinking and imagination), emotional (active and passive experiences), volitional (decision, execution, volitional effort, etc.).
Mental condition– a relatively stable level that has been determined at this time mental activity, which manifests itself in increased or decreased activity of the individual.
Every person experiences different mental states every day. With some one, mental or physical work is easy and productive, but with the other, it is difficult and ineffective.
The most studied:
- general mental state, for example attention, manifested at the level of active concentration or absent-mindedness;
- emotional states or moods (cheerful, enthusiastic, sad, sad, angry, irritable, depressed, inspired, creative state and so on.).
The highest and most stable regulators of mental activity are personality traits.
Under mental properties one should understand stable formations that provide a certain qualitative and quantitative level of activity and behavior typical for a particular person. They are synthesized and create complex structural formations of the personality, which include:
1) life position (a system of needs, interests, beliefs, ideals that determines a person’s selectivity and level of activity);
2) temperament (system natural properties personality (mobility, balance of behavior and activity tone), characterizing the dynamic side of behavior);
3) abilities (a system of intellectual-volitional and emotional properties that determines the creative capabilities of the individual);
4) character as a system of relationships and modes of behavior.
Mental personality traits include:
- temperament;
- direction;
- capabilities;
- character.
Mental processes, states and properties of a person are single manifestations of his psyche. Therefore, one and the same manifestation of the psyche can be considered in different respects. For example, affect as a mental property is general characteristics emotional, cognitive and behavioral aspects of the subject’s psyche in a certain, relatively limited period of time; as a mental process it is characterized by the stages of development of emotions; it can also be considered as a manifestation of an individual’s mental properties - hot temper, lack of restraint, anger.
V.A. Hansen, considers the categories process and state to be opposite, distinguishing them on the basis of dynamism. The current mental state, according to the author, is characterized by a set of parameter values of simultaneously occurring processes and is the background for them.
There are complex dialectical relationships between the two categories: mental processes under certain conditions can be considered as states. However, processes primarily perform the function of reflection, and states - the function of regulation.
According to A. O. Prokhorov, the autonomy of the categories processes and states lies in the temporal aspect, as well as in the conditioning of processes by mental states, which determine the range of their changes, features of deployment and method of organization.
Let's consider some characteristics of the mental state:
mental processes: 1 – sensations, 2 – clarity of perceptions, 3 – features of ideas, 4 – memory, 5 – thinking, 6 – imagination, 7 – speech, 8 – emotional processes, 9 – volitional processes, 10 – attention;
physiological reactions: 11 - temperature sensations, 12 - state of muscle tone, 13 - coordination of movements, 14 - motor activity, 15 - cardiovascular system, 16 - manifestations from the respiratory system, 17 - state of sweating, 18 - sensations from the gastrointestinal tract, 19 – condition of the oral mucosa, 20 – coloring skin;
experience scale: 21 - melancholy - cheerfulness, 22 - sadness - optimism, 23 - sadness - playfulness, 24 - passivity - activity, 25 - drowsiness - vivacity, 26 - lethargy - liveliness, 27 - intentionality of experiences, 28 - tension - liberation, 29 - heaviness – lightness, 30 – stiffness – looseness;
behavior: 31 - passivity - activity, 32 - inconsistency - consistency, 33 - impulsiveness - measuredness, 34 - thoughtlessness - thoughtfulness, 35 - uncontrollability - controllability, 36 - inadequacy - adequacy, 37 - relaxation - tension, 38 - instability - stability, 39 - uncertainty - confidence, 40 - closedness - openness.
Mental processes are directly related to mental states and properties. This relationship can be illustrated by the following table.
Table. Forms of manifestation of the human psyche
Processes |
States |
Properties |
|
Cognitive: |
sustained interest, creative enthusiasm, apathy, depression, etc. |
Prudence - carelessness |
|
Emotional |
boredom, sadness, joy, conflicting emotional states - stress, affect, frustration |
emotional stability / emotional instability |
|
mobilization readiness, initiative, purposefulness, decisiveness, perseverance, concentration, determination, restraint |
courage - timidity |
||
Motivations |
desires, aspirations, interests, drives, passions, doubt, uncertainty, confusion, confusion, fear, hope, the cognitive dissonance(“cognitive discrepancy”) |
Associated with externality: resignation, meekness, submission-obedience, unconditional submission to other people's demands, orders, compliance, complaisance, pliability to persuasion, reactivity. Associated with internality: initiative, stubbornness. Associated with decision-making: dogmatism, capriciousness, willfulness, tyranny, selfishness, indecision, frivolity, recklessness - decision-making not restrained by the arguments of reason (hence - reckless actions as a personality trait), irresponsibility, businessmanship, impulsiveness, greed, self-confidence, arrogance, self-will , willfulness, foresight, prudence, thoroughness, independence, riskiness |
|
There are also integral mental properties and formations, such as integrity or selflessness of the individual, etc. It is customary to highlight the socio-psychological properties of the individual (social intelligence, social competence, leadership style, etc. Objective personal characteristics: aloofness – responsiveness; consciousness - irresponsibility; gullibility - suspicion; diplomacy - straightforwardness; radicalism - conservatism, etc.
In modern psychology, there is a division of mental processes into three main subsystems:
- cognitive
- regulatory
- communicative.
The cognitive subsystem includes processes that ensure knowledge of the external environment and orientation in it ( cognitive processes: sensation, perception, representation, attention, imagination, memory, thinking); the second – processes aimed at building, organizing and regulating activity and behavior (volitional, emotional, motivational processes); the third is processes that ensure communication and interaction between people.
If we classify mental processes according to the degree of their integration, then we can distinguish three levels:
- cognitive, emotional, volitional and motivational processes
- regulatory (integral) processes
- reflective processes
Reflexivity acts as a meta-ability that is part of the cognitive substructure of the psyche, performing a regulatory function for the entire system, and reflexive processes - as “third-order processes” (considering cognitive, emotional, volitional, motivational processes of the first order, and synthetic and regulatory processes of the second order , which include goal setting, planning, forecasting, decision making, self-control, etc.). Reflection is the highest degree of integration process; it is at the same time a way and a mechanism for the mental system to go beyond its own limits, which determines the plasticity and adaptability of the individual.
In this approach, reflection is a synthetic mental reality, which is simultaneously a process, a property and a state. Reflection is at the same time a property uniquely inherent only to a person, and a state of awareness of something, and the process of representing one’s own content to the psyche.
As a human-specific ability, reflection is a fundamentally inherent ability to perceive not only the external, but also inner world. This is the ability to self-reflect one’s psyche, which is the basis of the property and phenomenon of consciousness. In addition, this is a kind of process of “thinking about thinking,” when the subject, the object of thinking, becomes itself. As a state, reflection is characterized by a person’s immersion in his thoughts and feelings, his detachment from surrounding events and phenomena.
Functional system
The theory of functional systems, proposed by P.K. Anokhin, changes traditional “organ” thinking and opens up a picture of holistic integrative functions of the body, i.e. postulates a functional approach to physiological phenomena.
Emerging from theory conditioned reflexes I.P. Pavlova, the theory of functional systems was her creative development. At the same time, in the process of development of the theory of functional systems itself, it went beyond the framework of the classical reflex theory and took shape into an independent principle of organization of physiological functions. Functional systems have a cyclic dynamic organization different from the reflex arc, all the activities of the constituent components of which are aimed at providing various adaptive results useful for the body and for its interaction with the environment and its own kind.
A functional system is a combination (coordinated activity) of nervous processes and organs of the human body that allows him to both effectively perform certain intended actions and correct their results (if they are incorrect), thereby adapting to the environment.
The mechanism for controlling actions and activities is considered in the most detail in P.K. Anokhin’s scheme, which is entirely applicable to voluntary control.
Any functional system, according to the ideas of P.K. Anokhin, has a fundamentally similar organization and includes the following general peripheral and central nodal mechanisms, which are universal for different functional systems:

Rice. Organization of a functional system according to P.K. Anokhin
The functional system includes the following elements: 1) control device - nerve center; 2) output channels through which communication with working organs is carried out (effectors) - nervous and hormonal regulation; 3) executive organs - effectors, ensuring, during physiological activity, the maintenance of the regulated process of the indicator at a certain optimal level (useful result of the activity of the functional system); 4) receptor systems that perceive information about the parameters of deviation of the regulated process of the indicator from the optimal level; 5) feedback channel (input channels) with the transfer of information from receptors to the nerve center.
The scheme for controlling human actions, according to P.K. Anokhin, includes five blocks:
A – block of afferent synthesis;
B – decision-making block;
B – block for drawing up a program of action or activity as a whole;
G – block of execution and obtaining results;
D is a feedback block that provides information about the results of the action taken.

Rice. Diagram of the functional system according to P.K. Anokhin.
OA – situational afferentation, PA – triggering afferentation
Let's consider how these blocks function and what is their contribution to the voluntary control of actions.
Afferent synthesis(from Latin afferens (afferentis) - bringing) - in the theory of a functional system (P.K. Anokhin) synthesis of material imprinted in memory, motivation, information about the environment and a trigger stimulus for the purpose of making a decision. Memory is interpreted as a set of interconnected functional systems of various levels of hierarchy, formed in the process of evolution and in individual life experience, and motivation is interpreted as a specification of one of the needs of the body. During afferent synthesis, thanks to motivation, all systems whose activity has ever led to the satisfaction of a given need are updated. Information about the environment helps to achieve the results required in a given environment. The final decision is made at the moment when some event - a triggering stimulus - gives an advantage to one of the systems already selected under the influence of motivation and environment. Due to the fact that the hierarchical organization of systems in memory reflects the evolutionary and individual history of the adaptive relationships of the organism with the environment, there is a corresponding hierarchy of afferent synthesis. Like anyone system process afferent synthesis does not take place in any separate brain structure, but is a process of interaction between neurons of the most diverse (central and peripheral, afferent and efferent) morphological affiliations throughout the entire brain and body.
Afferent synthesis, according to the theory of P.K. Anokhin, is carried out through the interaction of four factors: 1) triggering afferentation; 2) situational afferentation; 3) memory and 4) motivation.
Afferentation(from Latin afferentis - “bringing”) - a constant flow of nerve impulses entering the central nervous system from sensory organs that perceive information from both external stimuli (exteroception) and internal organs (interoreception). It is directly dependent on the strength of stimuli and the saturation of the environment with them, as well as on the state - activity or passivity - of the individual.
Situational afferentation- the sum of afferent excitations that arise in specific conditions and signal the situation in which the organism is located. Situational afferentation acts on an organism in which there is one or another level of motivational arousal (motivation).
Under triggering afferentation is understood as a push, i.e. a stimulus that, by revealing the excitation structure existing in the central nervous system, leads to the external appearance of any activity of the body. The success of a response action is a synthetic whole of situational and triggering afferentation and that specific gravity both can change depending on the developing living conditions of the organism. Experiments have shown that this constant organic synthesis of two types of afferentation is carried out with some participation of the frontal cortex cerebral hemispheres. By turning on reverse afferentation, the effect of the environment in which a given animal or person is located increases even more.
Reverse afferentation is an analogue of feedback in cybernetics and is of great importance for physiology and medicine. In any physiological process or behavioral act that is aimed at obtaining some kind of adaptive effect, reverse afferentation informs about the results of the action taken, allowing the body as a whole to assess the degree of success of the action performed.
Reverse afferentations that occur during any motor act are divided into two completely different categories: a) directing movement and b) effective afferentation. While the first afferentation is represented only by proprioceptive impulses from the muscles performing the movement, the second afferentation is always complex and covers all afferent signs relating to the very result of the movement undertaken. Both afferentations always have an organizing influence on the formation of subsequent actions of the organism. In fact, subsequent motor acts of the body will be directly dependent on the extent to which the reverse afferentation about the results of the action corresponds to the original stimulus.
The trigger signal is perceived by the senses in the form of sensations that send signals corresponding to the stimulus along conductors going to the nerve centers - afferent (sensitive) nerves. In the central nervous system, these signals are processed, as a result of which sensations are synthesized and the perception of objects and situations arises. “Recognition” of trigger information occurs with the help of long-term and short-term memory, i.e. traces of previous human activity in similar situations.
The processing of trigger information in the central nervous system has, first of all, the task of determining the significance of a given signal for a person. This is especially important in cases where several signals are received simultaneously and a person must choose which one to respond to now, which one later, and which one should not be responded to at all. However, before making a final decision, a person must compare the trigger afferentation with the situational (background) afferentation, which informs about the state of the person himself, about the external situation. If the situation interferes with obtaining the usual, standard reaction to a given stimulus, amendments are made to the program of action to achieve the goal.
Recognition of the trigger signal (which can also be a need) leads to the emergence of a “model of the needed future,” as N.A. puts it. Bernstein, i.e. models of what should happen in response to this stimulus.
Motivational arousal, arising on the basis of a need, contains in its architectonics the properties of those stimuli that lead to the satisfaction of this need: acting on cortical cells, it creates a special chemical “mood”. This mood of the cells determines their reaction, due to which sensory information is actively filtered. Thus, need arousal determines the active use and selection of special stimuli from the external world, signaling objects that can satisfy the body’s original need. This advanced reflection of the result of activity is formed on the basis of afferent synthesis.
Human activity is diverse both in meaning and action, and in the conditions in which it occurs. Different goals, objectives and operating conditions impose different demands on a person and his functional systems. Therefore, each time the program and operating conditions change, functional systems are partially or completely reorganized, i.e. may consist of a different number of blocks that perform their specific functions (each functional system involves different mental processes, motor and volitional qualities, etc.). This means that the architectonics (structure) of functional systems formed to obtain useful results (solving a problem) are different. Despite this, all functional systems, regardless of the level of their organization and the number of their components, have a fundamentally the same functional architecture and operating principles, which are understood as the laws of ordering the activities of subsystems in order to obtain a useful result.
Obviously, it is advisable to supplement these ideas of P.K. Anokhin with the ideas of A.A. Ukhtomsky about the dominant. According to these ideas, the dominant, as a temporarily dominant source of excitation (and need excitation can be called such) lowers the thresholds to adequate stimuli (corresponding to the dominant) and increases the thresholds to those stimuli that are not related to it. Consequently, the dominant contributes to the selective perception of stimuli, stimuli that signal the subject of need satisfaction.
Dominant motivation is formed on the basis of a leading need, with the participation of motivational centers of the hypothalamus. At the stage of afferent synthesis, the dominant motivation activates memory.
Integrated into the process of voluntary control, the above-described involuntary mechanisms for obtaining and processing information, independent of the will of a person, help make an informed decision, as if highlighting, like the rays of a spotlight, those objects and their properties that are necessary to satisfy a need.
Thus, “afferent synthesis” leads to a person receiving “information for thought,” i.e. information necessary to make an informed decision: what the goal should be, what are the external and internal conditions for achieving it.
Decision making is related to a person's confidence or uncertainty. This characteristic is expressed in conviction or, conversely, in a person’s doubt about the correctness decision taken. Confidence motivates a person to take action to implement the program; doubt forces a comprehensive check of the decision made. As a result, the action is delayed.
The degree of confidence is determined by a number of external and internal factors. The first includes information: the less information a person has and the more options that seem to be equivalent, the more (other things being equal) he feels more insecure. Factors such as an unexpected situation, a new environment, or lack of experience contribute to uncertainty. Internal (psychological) factors that cause uncertainty are anxiety, indecision as personal characteristics.
For some people (impulsive, passionate, with high self-esteem), confidence develops into overconfidence, which leads to forecasting without sufficiently careful consideration of all circumstances and one’s own capabilities. Such individuals, according to the observation of S.L. Rubinstein, as if deliberately putting themselves at the mercy of circumstances, being confident that the right moment will bring them the right decision. Therefore, it is believed that a certain degree of doubt and fear is even valuable, since this guarantees a certain margin of safety.
But, as N.A. Bernstein wrote, afferent signals often contain only information about “what is”, but not about “what needs to be done.” In this regard, the next stage of management is necessary: determining how, with the help of what available resources and means, the goal, the “required future” can be achieved. This is related to action programming.
Action programming. Programming of motor actions must, firstly, provide for the parameters of movements (spatial, speed, tempo, amount of required effort) and, secondly, the course of movements in detail. The first function correlates with the master mechanism, the second - providing a “kinetic melody” - with the programming mechanism (L. V. Chkhaidze). Both decision-making and programming are associated with a person’s ability to “look ahead,” i.e. extrapolate the future.
A particular type of extrapolation is anticipation, or a proactive response to some signals or moving objects.
Foresight in many cases cannot be absolute, but is probabilistic in nature: even with an unconditional and conditioned reflex response, the statistical apparatus of the brain calculates the most likely option for action that allows one to achieve a goal, or an option for responding to a signal.
The ability to compare incoming information about a current situation with information stored in memory about past experience and, based on all this data, build hypotheses about upcoming events, attributing to them one or another probability, is called probabilistic forecasting.
There is a distinction between objective and subjective probability. The first characterizes, for example, the frequency of occurrence of a particular situation. The second is the expected frequency of the event. Subjective probability may not correspond to objective probability. In the absence of information, when, for example, a person begins to perform an unfamiliar task, he proceeds from the conscious or unconscious assumption that events are equally likely; in reality, for example, one event may happen more often than others. This leads to the fact that at first a person makes many mistakes when forecasting. Gaining experience, he begins to bring the subjective probabilistic assessment of events closer to the objectively existing probability, as a result of which his behavior becomes adequate to the situation.
Memory involved in programming must store information not only about past events, but also about the likelihood of their occurrence, and the connections between occurrences various events. A certain role in probabilistic forecasting is played by emotions, which can compensate for the lack of information and, coloring the situation in one or another emotional background (pleasant or unpleasant), increase or decrease the subjective probability of a response.
Programming of actions and activities is carried out in three possible options: if there is complete information, in the presence of partial information and in the complete absence of information. These options correspond to probabilities ranging from one to zero. If the probability is equal to one, a strict program of activities is provided; There is no search as such. For example, a sprinter knows that when the starter fires, he needs to start running. In the absolute absence of information, probabilistic programming is useless, therefore, in the case of complete uncertainty, the search is carried out using the “trial and error” method, i.e. comes down to random (blind) obtaining a useful result (this corresponds to an external search for an object to satisfy a need. Scientists have different attitudes towards the last option for achieving a goal. Some consider it universal biological method adaptation, others consider it a special case and see its conditionality only in the absence of information. Obviously, this method must be assessed differentially, as W. Ashby did: if you see it as simply an attempt to achieve a goal, then this is really a “second-rate” method; if we consider it as an option for obtaining information necessary to achieve a goal, then this method can play a large role in acquiring experience.
In the intermediate option (when a person has incomplete information), which occurs most often, forecasting is difficult and is carried out in various ways:
1. A person prefers to act according to a “rigid” program.
2. He chooses several options and acts on one or another option.
3. He does not have a premeditated decision and acts depending on the situation, which requires high development tactical thinking.
At the end of programming, a signal to implement the program and the execution of the program itself (action or activity) follow. This stage in the figure corresponds to block G.
However, the management process does not end there. A person must know how the program is implemented step by step and as a whole and, in case of deviation from it, make corrections that return the system to the programmed direction. Control over actions is carried out using feedback and an acceptor of the result of the action (comparison apparatus).
So, the achievement of an adaptive result is carried out using specific mechanisms, of which the most important are:
1. afferent synthesis of all information entering the nervous system;
2. decision-making with the simultaneous formation of an apparatus for predicting the result in the form of an afferent model of the acceptor of the results of the action;
3. the actual action;
4. comparison, based on feedback from the afferent model of the acceptor, of the results of the action and the parameters of the performed action;
5. correction of behavior in case of discrepancy between real and ideal (modeled by the nervous system) action parameters.
The functional system according to P.K. Anokhin is a cybernetic scheme for controlling the body, aimed at achieving results useful for the body. The functional system characterizes the following properties of the behavior control scheme:
- purposefulness associated with the need to meet the needs of the animal;
- motivation, which sets the prerequisites (for example, due to needs) for the formation of a goal;
- a dominant that ensures the mobilization of the animal’s resources to achieve a priority goal, including the mobilization of intellectual resources (concentration of attention);
- situation recognition;
- “planning” actions;
- decision-making;
- forecast of the result of an action;
- performing the most purposeful action;
- assessment of the result of the action;
- comparison of forecast and result;
- finding the right solution and adjusting the knowledge base (in case of mismatch between the forecast and the result) - training.

Rice. Cybernetic diagram of a functional system (in the spirit of P.K. Anokhin)
Recognition, planning, decision-making are based on the use of a knowledge base, which is replenished during training.
An important concept of the functional system is motivation. The role of motivation is goal formation and support of goal-oriented forms of behavior. Motivation can be seen as active driving force, which stimulates finding a solution that is adequate to the needs of the animal in the situation under consideration. Motivation is closely related to the concept of dominance, which was introduced by A.A. Ukhtomsky. The dominant mobilizes a person’s resources to achieve a given goal. In particular, neural resources are mobilized so that the animal's attention is concentrated on the priority goal.
The composition of the functional system is not determined by the spatial proximity of the structures or their anatomical affiliation. It can include both nearby and distantly located body systems. It can involve individual parts of any anatomically integral systems and even parts of individual entire organs. In this case, a separate nerve cell, a muscle, a part of an organ, or the entire organ as a whole can participate through its activity in achieving a useful adaptive result only if it is included in the corresponding functional system. The factor determining the selectivity of these compounds is the biological and physiological architecture of the functional system itself, and the criterion for the effectiveness of these associations is the final adaptive result. The functional system is characterized by:
1. degree of plasticity, i.e. the ability to change their constituent components. For example, the functional system that ensures breathing consists predominantly of innate structures and therefore has little plasticity: the act of breathing, as a rule, involves the same central and peripheral components. At the same time, the functional system that ensures the movement of the body is plastic and can quite easily rearrange component relationships (you can reach something, run, jump, crawl);
2. individual and changing requirements for afferentation. It is the quantity and quality of afferent impulses that characterizes the degree of complexity, arbitrariness or automation of the functional system;
3. the ability for self-regulation, which is inherent in it as a whole. In the event of a possible defect in a functional system, a rapid restructuring of its constituent components occurs so that the desired result, even if less efficiently (both in terms of time and energy costs), is still achieved.
The initial stage of a behavioral act of any degree of complexity, and therefore the beginning of the functioning of a functional system, is afferent synthesis. The importance of afferent synthesis lies in the fact that this stage determines all subsequent behavior of the organism. The task of this stage is to collect the necessary information about various parameters of the external environment. Thanks to afferent synthesis, from a variety of external and internal stimuli, the body selects the main ones and creates the goal of behavior. Since the choice of such information is influenced by both the goal of behavior and previous life experience, afferent synthesis is always individual. At this stage, the interaction of three components occurs: motivational arousal, situational afferentation (i.e. information about the external environment) and traces of past experience extracted from memory. As a result of the processing and synthesis of these components, a decision is made about “what to do” and a transition occurs to the formation of an action program that ensures the selection and subsequent implementation of one action from many potential ones. The command, represented by a complex of efferent excitations, is sent to the peripheral executive organs and is embodied in the corresponding action.
A necessary part of the functional system is the acceptor of action results - the central apparatus for assessing the results and parameters of an action that has not yet taken place. Thus, even before the implementation of any behavioral act, a living organism already has an idea about it, a kind of model or image of the expected result.
In the process of real action, efferent signals go from the acceptor to the nervous and motor structures that ensure the achievement of the required goal. The success or failure of a behavioral act is signaled by afferent impulses entering the brain from all receptors that record the successive stages of performing a specific action (reverse afferentation). An assessment of a behavioral act, both in general and in detail, is impossible without such accurate information about the results of each action. This mechanism is absolutely necessary for the successful implementation of every behavioral act. Moreover, any organism would immediately die if such a mechanism did not exist.
The structure of the thinking process. Thinking is a process cognitive activity, in which the subject operates with various types of generalizations, including images, concepts and categories.
The appearance of speech in the process of evolution fundamentally changed the functions of the brain. The world of internal experiences and intentions has acquired a qualitatively new apparatus for encoding information using abstract symbols. This not only made it possible to transfer information from person to person, but also made the thinking process qualitatively different. We become more aware and understand a thought when we put it into linguistic form. Outside of language, we experience unclear impulses that can only be expressed in gestures and facial expressions. The Word acts not only as a means of expressing thoughts: it rebuilds the thinking and intellectual functions of a person, since the thought itself is accomplished and formed with the help of the word.
The essence of thinking is to perform certain cognitive operations with images in the internal picture of the world. These operations make it possible to build and complete a changing model of the world. Thanks to the word, the picture of the world becomes more perfect, differentiated, on the one hand, and more generalized, on the other. By joining the direct image of an object, the word highlights its essential elementary or complex features that are not directly accessible to the subject. The word translates the subjective meaning of the image into a system of meanings, which makes it more understandable to both the subject himself and his partner.
From the perspective of the theory of functional systems P.K. Anokhin, the main stages of the thought process can be compared with the stages of the structure of a behavioral act. The direction of the thinking process is determined by the dominant motivation of the subject. Afferent synthesis selects the search area for a solution to the problem. Incoming information is analyzed and compared with knowledge extracted from memory, the content of which is significantly determined by the dominant motivation. The decision-making stage corresponds to the selection of the most probable hypothesis for its subsequent testing and evidence. In the acceptor of the results of the action, in accordance with the accepted hypothesis, some ideas are formed about what should first of all be confirmed, proven or refuted. Efferent synthesis contains the intentions of evidence and verification. Carrying out a specific proof that confirms the validity of the assumption made is equivalent to the stage of carrying out the actual action. In case of failure, the subject's indicative research activity is activated. It leads to a change in the content of the acceptor results, as well as efferent synthesis. New plans, ideas arise and, perhaps, other methods of evidence are used.
There are two main types of thinking in humans; visual-figurative and verbal-logical. The latter functions on the basis of linguistic means and represents the most recent period of the phylogenetic and ontogenetic development of thinking.
Emotions. The functional system according to P.K. Anokhin does not take into account emotional processes. However, cognitive (cognitive) and evaluative operations influence emotions and are implemented in the brain, which is already emotional and is not affectively neutral. There is no purely cognitive determinant of emotions. Emotion to a significant stimulus is a unity of affective-cognitive processes.

Rice. Scheme of action formation
Emotions are an internal regulator of activity. However, emotions perform the function of regulating behavior not directly, but through motives, and often the motives of one’s own behavior remain unconscious to a person. This feature of emotional phenomena - their close connection with the sphere of the unconscious - also constitutes the most important specificity of emotions, which significantly distinguishes it from cognitive processes, which are largely carried out under the control of consciousness.
In the theoretical understanding of emotions, as is known, there are two extreme positions. On the one hand, these are biologizing ideas about emotions as an adaptive (and only) mechanism for adapting the psyche to the environment, on the other hand, these are intellectualistic ideas about emotions as a result of a lack of information. The first includes, for example, the concept of P.K. Anokhin, who did not see the difference between the emotions of animals and humans, either qualitatively or in terms of the functions they perform. An example of the second point of view is the information theory of P.V. Simonov, reducing all the diversity of emotions to a lack of information. Both concepts cannot lay claim to a holistic description of emotions as mental phenomena, although they reflect certain aspects of the emotional sphere. First of all, these concepts do not take into account the complex heterogeneous composition of emotional phenomena that make up the “emotional sphere” of a person. The “emotional sphere” of a person apparently includes various types of emotional phenomena, such as the “emotional tone of sensations,” an emotional reaction (or emotional process), emotional states, and emotional and personal qualities. Each of these types of emotional phenomena is characterized by its own patterns of formation, functioning and decay, which cannot be ignored when constructing a general psychological concept of emotions. The general psychological concept of emotions must also take into account the central factor for the human psyche - the factor of social experience, the cultural and historical determination of all human mental phenomena, including emotions. Social determination determines, first of all, the object (object) to which the emotional phenomenon is directed, i.e. emotional assessment of his perception. Social determination (through the type of mental activity) explains the emergence of a particular emotion. Cultural and historical determination also determines the forms of expression of emotions and the processes of their self-regulation. A general psychological theory of emotions should immanently include these aspects of emotional phenomena. Finally, the general psychological concept of emotions should also include ideas about the mechanisms of realization of emotions, i.e. about those psychophysiological patterns that ensure their implementation.
PC. Anokhin developed a biological theory of emotions, which emphasizes the adaptive nature of emotional reactions, their regulatory function in ensuring behavior and adapting the body to changing conditions environment. Anokhin identifies two main stages in the life activity of any organism: the stage of the emergence of a need and the formation of motivation and the stage of satisfaction of the need. Each of these stages is necessarily accompanied by emotions: the first - mostly negative, the second - mostly positive.
Emotions are the leading component of the brain’s information assessment of internal needs and the effects of external factors. If the problem of emotions is considered from a biological point of view, then it will be necessary to recognize that emotional sensations have become established as a kind of instrument that keeps the life process within its optimal boundaries and prevents the destructive nature of the lack or excess of any factors in the life of a given organism. Emotional level mental activity is genetically determined and does not require special training.
Negative emotions always arise and intensify in cases where there is a mismatch in the activity of a functional system: when metabolic needs arise and are not satisfied, when damaging factors act on the body, when information about the results achieved does not correspond to those programmed in the acceptor.
Positive emotions are formed in all cases when the subject achieves the required results. Based on repeated satisfaction of the same type of need, foresight is formed positive emotion when satisfying this need by including it in the acceptor of the result of the action.
It turns out that emotional reactions are one of the most important components of the learning process.
So, in accordance with the biological theory of emotions P.K. Anokhin, leading emotions with a negative sign signal the body about deviations in its internal environment (hunger, thirst), which activates the corresponding program of action. Completion of purposeful actions is accompanied by a positive emotional background, which is fixed in the animal’s memory as “receiving a reward.” Explaining his position, Anokhin gives an example when a predator purposefully pursues its prey for many days, which is accompanied by both negative experiences (feelings of hunger) and positive ones (the process of satiation). Thus: “leading emotions participate in the formation of a functional system, determining the vector, that is, the direction of behavior, goal setting, and the formation of an acceptor for the result of an action. Situational emotions that arise when assessing individual stages of action allow you to correct behavior and achieve your goal.”
Thus, the main information load in a biological theory is carried by its sign, which marks the program of behavior and gives it a certain direction.
The basis for considering the psyche as a single integral functional system in philosophy and psychology is the understanding of the psyche as a reflection of reality and the regulation of behavior and activity on this basis. From this understanding of the nature and purpose of the psyche, the question naturally followed: what exactly should be reflected in the psyche, what should be represented in it, so that behavior is adequate to external and internal conditions, and activity is successful. The system of basic mental processes necessary for successful behavior in the environment and for successful activity is built as follows:
1. The existing objective reality that exists in a given space at a given moment in time must be reflected.
2. Events that can take place in the future and take place in space beyond its immediate reality must be represented.
1 and 2 are cognitive processes that form cognitive subsystem of the psyche, including sensations and perceptions, anticipatory reflection of reality in the form of various kinds of anticipations and extrapolations, imagination, thinking.
3. The needs of one’s own body and personality must be reflected. This - need-motivational subsystem psyche.
4. The significance for the body and personality (positive or negative) of certain external factors, one’s own internal states, as well as the results of the interaction of the body and personality with the environment – natural and social – must be reflected in a direct, immediate sensory form. These are emotions and feelings that form emotional subsystem of the psyche.
5. It is imperative to have information about how reality is reflected in the psyche of other people: what they feel and perceive at the moment, what they know and understand, what they think about, what and how they foresee, what they feel, what their needs are, etc. P. Without taking into account information about the content of the psyche of other people (theoretically, all of them, but in specific acts of behavior and activity, of course, only some, depending on the circumstances), no behavior adequate to external conditions and no successful activity is simply impossible. At the same time, every person, if he wants the behavior and activities of other people to be somehow consistent with his own vision of the world, with your own feelings and needs, must convey to them the data of the contents of his psyche. These two-way processes of exchange of contents and states of people’s own psyches are carried out communicative subsystem of the psyche, including nonverbal and verbal-sign communication.
6. Of course, you need to take into account all past successful experience of reflecting and regulating behavior and activity. This - memory subsystem.
7. However, reflection processes are only one side of the matter, since life task psyche – to carry out behavior and activities adequate to the external environment and internal states of the subject. This means that synthesis and integration of all information coming from the six subsystems of the psyche mentioned above is necessary. This is carried out central, integration-volitional subsystem, where all information coming from other subsystems is synthesized, decision-making processes take place, goals, plans and behavior programs are developed.
8. Any mental activity requires the necessary activation-energy support for the work of all other subsystems, including the integration-volitional one. This provision is carried out activation-energy subsystem psyche. Moreover, the more difficult tasks and situations a person faces, the more demands are placed on the integral functional system of his psyche and its individual subsystems, the more (not necessarily linearly and, of course, up to a certain individually determined limit) its activation is turned on. energy subsystem.
The integration-volitional and activation-energy subsystems appear in evolution later after more or less relative differentiation of the remaining subsystems, when there is a need to harmonize and integrate their functions in the organization of adaptive acts (or cycles) of behavior. In the modern human brain, the highest integrative center is the frontal lobe of the cerebral cortex, the so-called prefrontal cortex. Its anatomical and functional connections indicate that it receives impulses from all subsystems of the developed functional system of the psyche:
1) projection and associative areas of the cortex (cognitive and anticipatory subsystems);
2) the hypothalamus and related structures (need-motivational subsystem);
3) limbic system (emotional subsystem);
4) hippocampus and related structures (memory subsystem);
5) speech areas of the cortex (speech communication subsystem);
6) reticular formation of the brain stem and other activating nonspecific structures (energy-activation substructure).
Function blocksbrain
Human mental processes are complex functional systems, and they are not localized in narrow, limited areas of the brain, but are carried out with the participation of complex complexes of jointly working brain apparatus, each of which makes its own contribution to the organization of this functional system. That is why it becomes necessary to find out what basic functional units the human brain consists of, how it is built and what role each of them plays in the implementation of complex forms of mental activity.
We can distinguish three main functional blocks, or three main brain apparatuses, the participation of which is necessary for the implementation of any type of mental activity. With some approximation to the truth, they can be designated as:
1) a block that provides regulation of tone and wakefulness;
2) a block for receiving, processing and storing information coming from the outside world;
3) block of programming, regulation and control of mental activity.
Each of these main blocks has a hierarchical structure and consists of at least three types of cortical zones built on top of each other: primary (or projection), where impulses arrive from the periphery or from where impulses are sent to the periphery, secondary (or projection-associative), where the received information is processed or the corresponding programs are prepared, and, finally, the tertiary (or overlap zones), which are the latest developing apparatus of the cerebral hemispheres and which in humans provide the most complex forms of mental activity, requiring the joint participation of many areas of the cerebral cortex.
1. Block for regulating tone and wakefulness. In order to ensure the full flow of mental processes, a person must be in a state of wakefulness. It is known that only under optimal waking conditions can a person receive and process information, recall the necessary selective systems of connections in memory, program his activities and control the course of his mental processes, correcting errors and maintaining the direction of his activities.
It is well known that in a state of sleep, clear regulation of mental processes is impossible, emerging memories and associations become disorganized, and directed selective (selective) performance of mental activity becomes impossible.
The fact that in order to carry out organized, purposeful activity it is necessary to maintain optimal tone of the cortex was also said by I.P. Pavlov, who hypothetically argued that if we could see how excitation spreads across the cortex of a waking animal (or person), we would observe “ a bright spot” that moves across the cerebral cortex as we move from one activity to another and represents the point of optimal arousal.
The development of electrophysiological technology has made it possible to see this “spot” of optimal excitation: with the help of a special device - the “toposcope” of M.N. Livanov (1962), which makes it possible to simultaneously record electrical activity in 50-100 points of the cerebral cortex - one can observe how in In the cerebral cortex of a waking animal, a “spot” of optimal excitation actually appears, how it moves during the animal’s transition from one state to another, and how, in a pathological state, it gradually loses its mobility, becomes inert, or completely fades away.
I.P. Pavlov not only pointed out the need for an optimal state of the cerebral cortex for the implementation of organized activity, but also discovered the basic neurodynamic laws of the emergence of such an optimal state. As has been shown by numerous studies of the Pavlovian school, the processes of excitation and inhibition occurring in the waking cortex obey the law of force and are characterized by a certain concentration, balance and mobility.
These basic laws of neurodynamics do not apply to states of sleep or fatigue. This is the result of the fact that in the so-called “inhibitory” or “phase” states, the tone of the cortex decreases and, as a result, the law of force is violated: weak stimuli are equal to strong ones in the intensity of the responses they evoke (“equalizing phase”) or even exceed them, causing more intense reactions than those caused by strong stimuli (“paradoxical phase”), in some cases reactions persist only in response to weak stimuli, while strong stimuli generally cease to cause any responses (“ultraparadoxical phase”) "). In addition, as the tone of the cortex decreases, the normal ratio of excitatory and inhibitory processes and the mobility that is necessary for normal mental activity are disrupted. All this indicates what crucial has the presence of optimal cortical tone for the organized flow of mental activity.
However, the question arises: what brain devices ensure the maintenance of optimal cortical tone, which we just talked about? What areas of the brain regulate and change the tone of the cortex, maintaining it at right time and increasing it when the need arises?
One of the most important discoveries in this regard was the establishment of the fact that the devices that provide and regulate the tone of the cortex may not be located in the cortex itself, but in the underlying stem and subcortical regions of the brain, and that these devices are in a dual relationship with the cortex, toning her and at the same time experiencing her regulating influence.
In 1949, two outstanding researchers - Magun and Moruzzi - discovered that in the stem parts of the brain there is a special nervous formation, which, both in its morphological structure and in its functional properties, is adapted to act as a mechanism regulating the state of the brain. bark, i.e. is able to change her tone and ensure her wakefulness.
This formation is built like a nervous network in which bodies are embedded nerve cells, connected to each other by short processes. Through the network of this formation, called reticular formation, excitation does not spread in separate, isolated impulses, not according to the “all or nothing” law, but gradually, gradually changing its level and, thus, modulating the state of the entire nervous system.
2. Block for receiving, processing and storing information. This block is located in the convexital (outer) sections of the new cortex (neocortex) and occupies its posterior sections, including the apparatus of the visual (occipital), auditory (temporal) and general sensitive (parietal) areas. According to its histological structure, it consists of neurons of the subcortex and cerebral cortex. These neurons, unlike the devices of the first block, do not work according to the principle of gradual changes, but according to the “all or nothing” law, receiving individual impulses and transmitting them to other groups of neurons.
The devices of this (as well as the next) block have a hierarchical structure, breaking up into primary (projection) zones, which receive information and split it into the smallest components, secondary (projection-associative) zones, which provide coding (synthesis) of these components and transform somatotopic projection into the functional organization, and tertiary zones (or overlap zones) providing working together various analyzers and the development of supramodal (symbolic) schemes that underlie complex forms of cognitive activity.
According to their functional characteristics, the devices of this block are adapted to receive exteroceptive stimuli coming to the brain from peripheral receptors, to crush them into a huge number of components (in other words, to analyze them into the smallest constituent details) and to combine them into the necessary dynamic functional structures ( in other words, to their synthesis into entire functional systems).
Thus, this functional block of the brain has high modal specificity: its constituent parts are adapted to receive visual, auditory, vestibular or general sensory information. The systems of this block also include the central apparatuses of taste and olfactory reception, but in humans they are so overshadowed by the central representations of higher exteroceptive, distant analyzers that they occupy an insignificant place in the cerebral cortex.
3. Block of programming, regulation and control of complex forms of activity. Reception, processing and storage of external information constitute only one side of a person’s mental life. Its other side is the organization of active conscious mental activity. The third of the main functional blocks of the brain is associated with this task - the block of programming, regulation and control of ongoing activity.
A person not only passively reacts to incoming signals. He forms plans and programs for his actions, monitors their implementation and regulates his behavior, bringing it into conformity with these plans and programs; finally, he controls his conscious activity, comparing the effect of his actions with the original intentions and correcting the mistakes he has made.
All this happens with the active participation of emotions. Emotion is a special form of mental reflection, which in the form of direct experience reflects not objective phenomena, but a subjective attitude towards them. The peculiarity of emotions is that they reflect the significance of objects and situations acting on the subject, determined by the relationship of their objective properties to the needs of the subject. Emotions serve as a connection between reality and needs. It can be argued that emotions arise as a result of exposure to a certain stimulus, and their appearance is nothing more than a manifestation of human adaptation mechanisms and the regulation of his behavior.
The processes of regulation and control of conscious activity require completely different brain apparatuses than the apparatuses of the first and second blocks. If even in simple reflex acts, along with the afferent side, there is an effector side and feedback devices serve as a control servomechanism, then such special controlling nerve formations are all the more necessary in complex mental acts. The devices of the third block of the brain serve these tasks. The apparatuses of the third functional block are located in the anterior sections of the cerebral hemispheres, in front of the anterior central gyrus.
Interaction of the three main functional blocks of the brain. It would be wrong to think that each of these blocks can independently carry out one or another form of activity, considering, for example, that the second functional block fully carries out the function of perception and thinking, and the third - the function of movement and construction of actions.
Having accepted the position about the systemic structure of complex psychological processes, we must take a different point of view. Each form of conscious activity is always a complex functional system and is carried out based on the joint work of all three blocks of the brain, each of which contributes to the implementation of the mental process as a whole. The facts, which are well established by modern psychology, make this position indisputable.
The time has long passed when psychologists viewed mental functions as isolated “abilities,” each of which could be localized in a specific area of the brain. Another concept was also rejected, according to which mental processes were represented according to the model of a reflex arc, the first part of which was purely afferent in nature and performed the functions of sensation and perception, while the second - effector - part entirely carried out movements and actions.
Modern representations the structure of mental processes is based on the model of a reflex ring or a complex self-regulating system, each link of which includes both afferent and efferent components and which, in general, has the character of complex and active mental activity.
Let's look at two examples: perception and movement, or action. Let's do this only in the most general terms.
It is known that sensation includes motor components, and modern psychology considers sensation, and especially perception, as a reflex act containing both afferent and efferent links; To be convinced of the complex active nature of sensations, it is enough to recall that even in animals they include the process of selection of biologically significant features, and in humans they also include the active coding influence of language. The active nature of processes appears even more clearly in complex objective perception. It is well known that object perception is not only multireceptor in nature, relying on the joint work of a whole group of analyzers, but always includes active motor components. The decisive role of eye movements in visual perception was noted by I.M. Sechenov (1874–1878), but this was only proven recently. In a number of psychophysiological studies, it has been shown that a stationary eye is practically unable to perceive an image consisting of many components, and that complex object perception involves active, searching eye movements that highlight the necessary features, and only gradually, as it develops, takes on a collapsed character.
All these facts convince us that perception is carried out with the joint participation of all those functional blocks of the brain, of which the first provides the necessary tone of the cortex, the second carries out the analysis and synthesis of incoming information, and the third provides directed search movements, thereby creating the active nature of perceptual activity .
It is precisely this complex structure of perception that explains why its disturbances can occur when various brain apparatuses located far from each other are affected. The same can be said about the construction of voluntary movement and action.
The participation of efferent mechanisms in the construction of movement is self-evident; however, still N.A. Bernstein (1947) showed that movement cannot be controlled by efferent impulses alone and that its organized flow requires constant afferent processes that signal the state of the joints and muscles, the position of the segments of the moving apparatus and the spatial coordinates in which the movement occurs.
Thus, voluntary movement, and especially objective action, is based on the joint work of the most diverse parts of the brain, and if the devices of the first block provide the necessary muscle tone, without which no coordinated movement would be possible, then the devices of the second block make it possible to carry out those afferent syntheses, in the system of which movement occurs, and the devices of the third block ensure the subordination of movement and action to the corresponding intentions, create programs for performing motor acts and provide that regulation and control of the flow of movements, thanks to which its organized, meaningful character is preserved.
FEDERAL AGENCY FOR EDUCATION
State Educational Institution of Higher Professional Education “Pomeranian State University named after. M.V. Lomonosov"
KORYAZHEMSKY BRANCH
FACULTY OF CHEMISTRY AND GEOGRAPHY
Department of Chemistry
METHODOLOGICAL APPROACHES TO FORMING KNOWLEDGE ABOUT CHEMICAL REACTIONS
course work
Protected with the mark _______________
Scientific director _____________
Koryazhma
Introduction
Chapter 1. The structure of the concept of “chemical reaction” and its stages
formation
1.1 The concept of “chemical reaction” as a system
1.2 Stages of formation of the concept of “chemical reaction”
Chapter 2. Basic methods used in the sections on chemical
2.1 Introduction of the concept of “chemical reaction”
2.2 Formation of knowledge on types of chemical reactions
2.3 Formation of knowledge about ion exchange reactions
2.4 Formation of knowledge about chemical kinetics
Conclusion
Bibliography
Application
Introduction
The topic of this course work is “Methodological approaches to the formation of knowledge about chemical reactions.” A methodical approach, or a method, is a way of achieving a goal, an activity ordered in a certain way. The main goal that a chemistry teacher must achieve when studying this concept is to form an entire system of knowledge about chemical reactions, consisting of separate subsystems and blocks of knowledge. Students must not only master the theoretical material of this topic, but also be able to apply the acquired knowledge in practice, understand the chemical processes that form the basis of chemical production (production of sulfuric acid, mineral fertilizers, etc.) and the chemical phenomena that constantly occur in nature (changes in the mineral composition of rocks, the formation of ozone in the atmosphere), understand the importance of using the safest methods for obtaining new alternative building materials for the environment.
This topic is relevant, since it is necessary to develop the most effective methodological approaches to the formation of knowledge about chemical reactions that satisfy the goal.
The object of the research is a theoretical system of knowledge about a chemical reaction, and the subject is those methodological approaches that contribute to the effective understanding and assimilation of knowledge about a chemical reaction.
The purpose of the work is, first of all, to consider the system-forming concept of “chemical reaction”, study and analyze the approaches used in the formation of the main blocks of knowledge about chemical reactions.
Here it is important to study the main subsystems integrated by the general concept of “chemical reaction”, show the connections between them, consider the properties of this system, reveal the stages of formation of this concept as students accumulate theoretical material, describe the methods (their content) used at the modern level of teaching chemistry ( general logical, general pedagogical, specific), show their application in combination when studying sections on chemical reactions.
Chapter 1. The structure of the concept of “chemical reaction” and the stages of its formation
1.1 The concept of “chemical reaction” as a system of content of an educational subject
The system of concepts about a chemical reaction is a very complex, multifaceted, multicomponent system. This complicates the generalization of knowledge and the identification of an invariant of a given system of concepts. In a developed and structurally formulated form, the general concept of a chemical reaction represents a theoretical system of essential knowledge about it. The scientific and theoretical foundations of its formation are the theories of the structure of substances and chemical processes, the periodic law and the law of conservation of mass and energy. The concept of “chemical reaction” is closely related to the concept of “substance”. This is a reflection of the dialectical connection between the type of matter and the form of its movement. During chemical reactions, substances are transformed. Chemical reactions are phenomena in which the composition, structure and properties of chemical compounds change - some substances are converted into others.
The leading idea for the successive formation and generalization of knowledge about chemical reactions in school should be the triune structural-energetic-kinetic approach, since from these positions it is possible to give a versatile characterization of the reaction.
The basis for deploying the entire body of knowledge about a chemical reaction in the form of a theoretical system is the genetically initial relationship between the reagents and reaction products. The genetically initial relation lying at the center of this knowledge system reflects the general model of a chemical reaction:
REAGENTS→REACTION PRODUCTS
where PAK is the transition active complex.
Essential features and aspects general concept chemical reaction are the following blocks of knowledge:
a block of knowledge about the conditions and signs of reactions;
block of knowledge about the energy of chemical reactions;
block of knowledge about the kinetics of chemical reactions;
block of knowledge about chemical equilibrium;
block of knowledge about the laws of reactions.
The fundamental concepts of this system are “reactivity”, “transition state”, “reaction rate”, “reaction mechanism”. These concepts are at the center of modern theoretical chemistry like nodal ones. Therefore, the leading approach in the analysis and formation of this system is the kinetic approach.
The essence of the chemical reaction is the formation of PAA according to the scheme:
initial state – transition state – final state of the reaction system. As V.I. Kuznetsov writes: “The transition state of a system is the essence of chemical transformations, the essence of any chemical process.” During chemical reactions, bonds in the starting substances are broken and others (usually stronger and more energetically favorable) are formed in the reaction products.
The elementary substance of a chemical reaction are atoms (ions, radicals) of elements. The conservation of atoms and their fundamental properties, including their masses, charges, etc., serves as the basis for quantitative descriptions of chemical reactions, for establishing quantitative relationships reflected by reaction equations. This explains their submission to the law of conservation of mass and energy. The restructuring of the electronic structures of atoms, molecules and other particles participating in the reaction that occurs during the transformation of substances is accompanied by the formation and transformation of chemical energy into its other types. The energy sign is one of the most important signs of a chemical reaction.
All this essential knowledge, reflecting the characteristics, aspects, connections and relationships of a chemical reaction, constitutes the theoretical core of the system of concepts about a chemical reaction. This system can be represented by the following diagram:
|
Substance Knowledge |
2. Conditions arise ia and leakage reactions and them signs |
3. Mechanism reactions |
4. Speed reactions |
chemical production |
|||
|
Reaction model |
5. Chemical equilibrium |
||||||
|
Reagents products initial final state state final state |
|||||||
|
1. Reaction Naya ability substances and energy processes |
6. Chemical law noi and control chemical reactions |
||||||
|
classification of chemical reactions |
|||||||
|
Electrondi- namical |
Electronic static |
||||||
|
7. Reaction equations |
|||||||
Fig.1. System of knowledge about chemical reactions in a school chemistry course.
1. The block of knowledge about the conditions and signs of reactions includes mainly empirical concepts formed on the basis of experiment and observations. Signs of reactions are identified on the basis of experimental data. Comparison of experiments makes it possible to identify common features for all reactions - the formation of new substances and energy changes that accompany these changes.
2. The block of knowledge about the energy of chemical reactions allows you to answer the question of why chemical reactions occur, whether they are possible or impossible, and what are the driving forces of the reactions. In a school chemistry course, energy knowledge is represented by such elements of thermochemistry as the thermal effect of a reaction, thermochemical equations; In high school, the concepts of entropy and Gibbs energy are introduced. In addition, they include the concept of activation energy.
3. The block of knowledge about the kinetics of chemical reactions answers the question of how chemical reactions proceed, reveals the course of the reaction over time, and their mechanism. This problem is central to modern chemistry, therefore, when considering reactions, the kinetic approach is the leading one, including in school.
The most important concepts of this block are: “reactivity”, “reaction rate”, “activation energy”, “activated transition complex”, “reaction mechanism”, “catalysis and its types” and others. In addition, this block includes such laws as Van't Hoff's rule, the law of mass action (without taking into account stoichiometric coefficients or for reactions where these coefficients are equal to 1). The most common concept is “reactivity”. It reveals the connection between the properties of reagents and various factors, including kinetic ones.
The concept of the rate of a chemical reaction characterizes the course of a reaction over time, reflecting the nature of changes in the properties of the reagents and their concentrations. It is determined by the change in the concentration of reacting substances per unit time. Reaction rate is a central concept in the system of knowledge about reactions in a school chemistry course. Its main purpose is a qualitative and quantitative description of the course of reactions over time.
The concept of “reaction mechanism” is the most abstract and difficult to understand. Therefore, first we give its simplest formulation: the reaction mechanism is a sequence of elementary chemical acts. This concept reveals the course of a chemical process, both in time and in space (number of particles, sequence of collisions, structure of PAA). Taken together, the concepts of “reaction rate,” “reactivity,” and “reaction mechanism” form the core of kinetic knowledge. The factor connecting them is the concept of an “intermediate activated complex,” which reflects the unity of stability and variability of chemical compounds, the mechanism of many reactions. The activated complex is characterized as an unstable intermediate with a large amount of energy and as an intermediate state of the reaction. This concept is closely related to the concept of “activation energy” - the optimal energy that reacting particles (molecules, ions, etc.) must have so that upon collision they can enter into a chemical reaction.
4. Block of knowledge about chemical equilibrium.
The most important concepts of the block are: “forward and reverse reactions”, “chemical equilibrium”, “factors and patterns of displacement of chemical equilibrium”. The theoretical basis for the disclosure of this material is the basic principles of kinetics and thermodynamics, Le Chatelier's principle and others. The integrative concept of this block is chemical equilibrium. Traditionally, knowledge about chemical equilibrium is included in the system of concepts about kinetics, and is considered as the equality of the rates of forward and reverse reactions. Consideration chemical equilibrium from this position it is one-sided. A thermodynamic approach to considering this issue is also possible. Here, chemical equilibrium is considered as a balancing of enthalpy and entropy factors, as the equality of two opposite tendencies - to order and disorder, taking place in a closed system at a constant temperature and constant amounts of reagent substances.
5. The block of knowledge about the laws of reactions reveals repeating connections and relationships between objects and phenomena of chemistry. These patterns include:
regular ratios of masses of reagents and reaction products, ratios of volumes of reacting substances (for gases);
the course of reactions towards a decrease in the free energy of the system (∆G
the dependence of the reactivity of substances (bonds, atoms, ions) on the electronegativity and degree of oxidation of the atoms of the elements included in their composition;
dependence of the reaction on the nature of the reagents;
dependence of the reaction rate on various factors (concentration of reagents, their state and particle size, temperature, pressure, etc.);
dependence of the shift in chemical equilibrium on kinetic factors (changes in temperature and pressure, concentration of reacting substances).
An important accumulator of chemical laws is the periodic system of D.I. Mendeleev; many of the laws are generalized by the electrochemical series of metal voltages.
This theoretical system of knowledge has the functions of description, explanation and prediction. This level of development is achieved by this system at certain stages of training as a result of theoretical generalization and application of knowledge. Passing in its development through successively changing theories, enriched with new knowledge and skills, it acquires the structure and functions of theoretical knowledge systems.
includes primarily empirical concepts formed on the basis of
1.2 Stages of formation of the concept of “chemical reaction”
Due to the fact that the concept of a chemical reaction is quite complex and multifaceted, it is impossible to form a complete understanding of all its sides and reveal its entire philosophical essence in a short period of time. Moreover, this concept is formed throughout the entire chemistry course.
The concept of “chemical reaction” is formed in stages.
First stage (8th grade). In the initial stages of studying chemistry, an inductive approach is used. The basis of the study of how the source of chemical knowledge lies chemical experiment. As a result of observing the experiment, students become aware of the formation of new substances during a chemical reaction. But in the experimental study of reactions, no attention is paid to its essence, the emphasis is on external manifestations (change in the color of the solution, gas release, precipitation).
The concept of a chemical reaction begins to form from the very first lessons. First, they give an idea of the phenomena occurring in nature, everyday life, and everyday life, distinguishing between physical and chemical phenomena. And then they inform students about the identity of the concepts “chemical phenomenon” and “chemical reaction”. At the level of atomic-molecular teaching, they explain how one can detect the occurrence of a chemical reaction by external signs.
The classification of chemical reactions is given at the level of comparing the number of starting and resulting substances. At the same time, students use such mental techniques as comparison, analysis, synthesis, and generalization. All of this information is included in the "Initial Chemical Concepts" section. Next, all aspects of the system of concepts about a chemical reaction must be expanded and supplemented with new data, i.e., the accumulation stage begins. The patterns of chemical reactions are analyzed using the simplest examples: the influence of temperature is considered on the reaction of the formation of iron sulfide, oxidation reactions are considered as the process of combining a substance with oxygen, the concept of exchange reactions using the example of the interaction of acids with oxides, etc.
At the second stage (grade 8), the concept of a chemical reaction is further developed. Energy ideas about chemical reactions begin to form. The concept of exo- and endothermic reactions is considered, a new concept is introduced about the thermal effect of a chemical reaction, thermochemical equations and their composition. When studying energy effects, it becomes possible to show not only the qualitative, but also the quantitative side of a chemical reaction. Quantitative ratios of substances that entered into a reaction are interpreted as molar ratios of reacting substances.
At the third stage (8th grade) of formation, the concept of “chemical reaction” undergoes qualitative changes in the topic “Chemical bond. The structure of matter." In this topic, a chemical reaction begins to be interpreted as the destruction of some bonds and the formation of others. This is considered using the example of redox reactions. The mechanism of these reactions is explained in terms of electron transfer, thereby rising to a higher theoretical level.
Based on the new concept of “oxidation state,” reactions of different types known to students are analyzed, thereby proving that redox reactions can be found among reactions of any type.
The topic “Oxygen subgroup” introduces a new concept of allotropy and a new type of reaction corresponding to it - allotropic transformations.
Fourth stage (9th grade). In the section “Regularities of chemical reactions,” the concept of the rate of a chemical reaction and the factors influencing it (temperature, concentration, contact surface) is introduced. The issue of the reversibility of a chemical reaction and chemical equilibrium is also considered here. It is necessary to emphasize the dynamic nature of chemical equilibrium and the factors that cause a shift in chemical equilibrium. Thus, students are introduced to another type of chemical reaction - reversible.
Stage five. At this stage, students are introduced to such an important topic as “The Theory of Electrolytic Dissociation.” In addition to its ideological significance (illustration of the unity and struggle of opposites - molarization and dissociation), it introduces a lot of new things into the explanation of the mechanism of reactions. On the basis of the concept of reversible reactions, it is possible to explain the essence of the dissociation process, as well as the hydrolysis of salts, considered in ionic form, so as not to introduce the concept of hydroxo salts.
Stage six (grades 9 – 10). Further development of the concept of a chemical reaction is carried out in the course of organic chemistry. The concepts of the classification of chemical reactions are supplemented, new types of reactions are introduced, for example, isomerization, polymerization, esterification, etc. In organics, a qualitative new material and the concept of reaction mechanisms. For example, the free radical mechanism is considered using the example of substitution reactions (halogenation of alkanes), addition (polymerization), and elimination (cracking). The concept of the ionic mechanism of a chemical reaction is expanded: examples of the addition of inorganic compounds to alkenes and substitution reactions during the hydrolysis of haloalkanes are given.
The system of concepts about the patterns of chemical reactions is also supplemented. When developing the concept of “rate of a chemical reaction,” the influence of bond energy and its type is noted. Knowledge about catalysis and catalysts is complemented in organics by knowledge about enzymes.
Stage seven (grade 11). At the final stage of training, results are summed up and knowledge about chemical reactions is generalized. At the end of the training, students should be able to characterize the chemical reaction given to them as an example in the light of the components of its content.
Chapter 2: Basic Techniques Used in Chemical Reaction Sections
2.1 Introduction of the concept of “chemical reaction”
The very definition of chemistry gives the subject of study - chemical phenomena accompanied by the transformation of substances. Students should not just memorize this definition, they must first understand the subject and in the learning process it should be constantly emphasized. When forming knowledge about chemical phenomena, it is important to take into account such a principle of dialectics as the transition from abstract to concrete knowledge. The foundation of such training will be the original concept of science, i.e. abstraction. To rely on a concept means to derive its specific, particular forms from the universal.
Together with the teacher, students carry out quasi-research subject activities and discover the subject of knowledge of chemistry - a chemical phenomenon. The process of cognition is based on analysis, reflection and prediction of available experiments, only some of which are carried out by the teacher, and the majority by the students themselves.
So, with the help of a teacher, they analyze what is happening in the world around them and discover the occurrence of various phenomena. Students reproduce some of them experimentally. The experimental results indicate changes in substances - this is a sign of any phenomenon. Taking the nature of changes in substances as the basis for classification, phenomena can be divided into two groups. The first includes phenomena in which only the transition of substances from one state to another occurs, and the second includes the transformation of some substances into others. The first group of phenomena is called physical (schoolchildren study them in a physics course), the second group is called chemical (students encounter them for the first time).
To more clearly differentiate the phenomena considered, as well as other phenomena proposed by the students themselves (for now based on their main external features), schoolchildren model them in graphic or symbolic form (optional). Subsequent analysis of models and comprehension of generalized phenomena according to the “was-has become” scheme shows students that with physical phenomena what was, remains, that is, substances did not change their nature, but only passed into another state, whereas with chemical phenomena it was something one thing, but it became something else.
The implementation by students of the actions described above allows them to identify a universal feature of chemical phenomena (in comparison with physical ones) - the transformation of substances - and thereby discover the subject of chemistry. On the basis of this same universal characteristic, an abstract (i.e. one-sided) definition of the concept “chemical phenomenon” is formulated at the level of representation: a chemical phenomenon (chemical reaction) is the process of transforming some substances into others.
Thus, from the very beginning of teaching chemistry, the teacher introduces students to the situation of discovering a new property of reality for them - the transformation of substances, characterized by the as yet unknown abstract concept of “chemical phenomenon (chemical reaction).”
To motivate students to further study chemistry, the teacher, when discussing issues of chemical phenomena, asks them to think: are chemical phenomena important in nature, in industrial production, in human life? Why do you need to study them? After their discussion, students begin to study the subject of chemistry - the transformation of substances. Students can easily differentiate the phenomena they are familiar with into physical and chemical, but if they are shown, for example, the process of dissolving sugar and the interaction of solutions of hydrochloric acid and alkali, then they are unlikely to be able to unambiguously attribute the latter process to chemical phenomena (there are no visible signs of a reaction). Thus, the teacher leads students to the idea that external signs alone are not enough to call a phenomenon chemical.
In this regard, the teacher sets an educational task: to identify internal signs of the transformation of some substances into others.
A new stage of quasi-research by students begins, aimed at logical abstraction, dividing the subject of research into components. At this stage, students explore the internal structure of the concept of a chemical reaction.
To do this, the teacher suggests studying the substances involved in transformations. Together with the students, the teacher formulates a hypothesis: perhaps the essence of the reaction lies in the study of the substances involved in it. To solve this problem, it is necessary to use abstraction, that is, mentally extract models of chemical phenomena, and experimentally study real substances. Learn to create new models of substances. These actions make it possible to transfer students’ thinking to an abstract level of understanding of substances, thereby concretizing the concept of “chemical phenomenon.”
The most appropriate way to study a substance is through observable signs, but if they are not there, it is necessary to somehow influence the substance. Students already know that substances are made up of atoms linked into molecules. In some substances the bonds are stronger, in others less strong. The hypothesis is again put forward: if substances consist of microparticles, then transformations may consist of changes between molecules and bonds. With a change in the hypothesis, a new educational task is formulated: to find out what happens to microparticles and the bonds between them during the chemical transformation of substances.
Thus, the mental activity of students is transferred to the micro level of organization of matter.
In accordance with the principles of activity and objectivity, students’ mental actions should be based on the results of experiments.
Students are shown a simple experiment: heating water, its subsequent evaporation and condensation. When heated, the bonds between water molecules are broken, since when energy is imparted to them, their mobility increases. When steam condenses, bonds are formed again between water molecules. Schoolchildren conclude that no changes occurred in the process of breaking and forming bonds between molecules, which means this is a physical phenomenon.
Thus, having studied the phenomena between substances, only atoms remain unstudied.
A hypothesis is again put forward: perhaps the essence of the transformations of substances lies in the changes that occur with atoms and the bonds between them. And again, the educational task changes - to find out what happens to atoms of different types and to the bonds between them during the transformation of one substance into another, and how this can be established. The teacher demonstrates the electrolysis of water, during which oxygen and hydrogen are formed. By modeling this process, students see: decomposition is accompanied by the breaking of bonds in a water molecule, and then the formation of bonds between two oxygen atoms and four hydrogen atoms.
Thus, students realize that chemical phenomena occur at the level of consideration of atoms and the bonds between them.
After modeling other chemical processes and identifying their general characteristics, students draw a conclusion: the essence of a chemical phenomenon (reaction) lies in the breaking of bonds in the starting substances and the formation of new bonds between atoms of the same types in the reaction products. Now they can formulate a definition of a chemical phenomenon at the level of an abstract entity: a chemical phenomenon is the process of breaking bonds between particles of initial substances and the formation of new bonds in the reaction products between the same particles, but in a different combination. This definition is abstract for students simply because students cannot answer the question of why some connections are broken while others are formed. To answer this question, students need to first learn about atoms and then the bonds between them.
After studying atoms, students can construct chemical compounds, first at the micro- and then at the macro-level of the organization of matter, and only then, knowing the strength of bonds in substances, comprehend and predict the processes of their breaking and formation.
As each level of organization of a substance associated with chemical phenomena is studied, the concept of “chemical reaction” becomes more and more specific.
The method of setting hypotheses and searching for answers to them, understanding the occurring phenomena constitutes the stage of schoolchildren’s entry into the oriented-motivational process, which is important for transferring the student from the position of an object of influence to the position of a subject who himself collaborates with other students and teachers. Students who have reached this stage can consciously answer the questions: what does chemistry study? Why should it be studied? What is the way to know it?
When searching for the answer to the first question, students open the subject of chemistry; responding to the second, they update the internal motives and needs of its study; discussing the third, they comprehend the plan for studying chemistry (at an abstract level) in accordance with the principle of ascent from the abstract to the concrete.
As a result, we can say that if students comprehend the dialectically structured content of educational material, discovering the principles and laws of dialectics and use them as a means of orientation in the world and knowledge of the surrounding reality, then we can probably state the fact of the formation of a personality with a developed dialectical way of thinking .
2.2 Formation of knowledge about types of chemical reactions
The study of atomic-molecular science and initial chemical concepts, as well as some accumulation of facts, allows a more meaningful approach to the classification of reactions.
The first acquaintance with the classification of substances shows that it is based on their composition and properties: substances are divided into simple and complex (based on composition), and simple substances are divided into metals and non-metals (based on properties).
Thus, any classification of phenomena, objects, substances is associated with the choice of some essential features that can be used as the basis for dividing objects or phenomena into groups.
Is it possible to classify chemical reactions? What is the basis for their classification?
The essence of any chemical reaction is to change the composition of the molecules of the substances taken for the reaction. Therefore, the nature of these changes must form the basis for the classification of chemical reactions. After explaining the problem posed to the students, you can ask them to name the reactions they know and write the equations of these reactions on the board.
|
H2O=H2+O2 |
In some cases, from the molecules of one substance, 2 molecules of other substances are obtained - these are decomposition reactions, in others, on the contrary, from the molecules of two substances one molecule of a new substance is formed - these are compound reactions. The teacher, together with the students, analyzing these conclusions, finds out whether molecules of one complex substance are always formed from molecules simple substance. To answer this question, the teacher carries out a decomposition reaction, for example, of malachite or potassium permanganate.
Thus, students realize that during the decomposition of complex substances, both complex and simple substances (or a mixture of both) can be formed. In conclusion, students sketch a diagram of this experiment, make the necessary notes on the drawing and write down the reaction equations.
Further, when forming students' understanding of the types of reactions, the teacher again puts forward the problem: can any other rearrangements of atoms occur during a chemical reaction other than those that occur during chemical reactions of addition and decomposition?
To answer this question, the teacher demonstrates to the students an experiment between a CuCl 2 solution and iron (iron nail). During the process, the iron nail is coated with a coating of copper. The teacher asks the question: can this reaction be classified as a compound or decomposition reaction? To answer this question, the teacher writes the reaction equation on the board (thus connecting the model of the process with the real experiment just carried out) and explains that this reaction cannot be attributed to either type, since during the process the molecules of two substances two molecules of new substances are also formed. This means there is reason to identify another type of reaction. This is the third type of chemical reaction, which is called displacement. It must be emphasized that the substitution reaction involves one simple and one complex substance.
At the end of the lesson, students complete a series of exercises on this topic, thereby acquiring and consolidating skills in working with new material. In addition, students are given homework on this topic.
As can be seen from the above, during the lesson the teacher, when explaining this material, uses the methods of conversation, story, and explanation. Thanks to leading questions, students are included in the thinking process. Here it is rational to use clarity, in which the leading role is given to the chemical experiment. It is important to connect the types of reactions with processes occurring in life (for example, the process of release of copper on an iron nail indicates its destruction; this process of metal destruction is present everywhere).
After introducing the exchange reactions, the teacher again offers to discuss two reactions. These could be, for example, the following:
Mg + H 2 SO 4 = MgSO 4 + H 2 and MgO + H 2 SO 4 = MgSO 4 + H 2 O.
What are the similarities and differences between these reactions? Discussing these process models with the teacher, students should come to the following conclusions:
the similarity is manifested in the fact that the amount of starting materials and reaction products is the same; one of the products in both cases is the salt MgSO 4;
difference: the starting substances of one of the reactions are complex substances, in the other - simple and complex;
reactions are of different types.
Having received these answers, or leading students to them with leading questions, the teacher suggests considering two more reactions:
FeO + H 2 SO 4 = FeSO 4 + H 2 O and FeCl 2 + H 2 SO 4 = FeSO 4 + 2HCl.
Again, during the discussion, students come to the following conclusions:
the substances involved in the reactions belong to different classes of inorganic compounds (FeO - basic oxide and acid, FeCl 2 - salt and acid);
in these reactions, complex substances exchange constituent parts (atoms or groups of atoms);
reactions are of the same type.
Reactions between complex chemical substances that result in exchange between atoms or groups of atoms are called exchange reactions.
As a special case of exchange reactions, the teacher needs to tell students about neutralization reactions. After reading and writing down the following rules indicating the possibility of a reaction:
During the reaction, water is formed;
a precipitate appears;
gas is released;
Students outline the characteristic features of exchange reactions:
CuSO 4 + NaOH, HCl + K 2 CO 3, NaOH + HCl.
The study is carried out as follows:
writing reaction equations,
working with the solubility table,
conclusion about the possibility of the reaction occurring,
experimental verification.
After conducting an experimental test, students note the absence of visible signs of the last reaction. The teacher explains that this reaction is a neutralization reaction, and reactions of this type must be carried out in the presence of indicators, by the change in color of which it is necessary to judge that the reaction has taken place.
Thus, students receive, on the basis of atomic-molecular teaching, a first understanding of the classification of reactions. Subsequently, the idea of classification formed at this level undergoes a number of qualitative and quantitative changes and additions. Thus, there is an increase in the study of the quantitative side of processes (the law of conservation of mass, Avogadro’s law and consequences from it, etc. are being studied). In the quantitative description of chemical reactions, the study of the elements of thermochemistry contributes to the prediction of the possibilities of their occurrence: the thermal effect, thermochemical equations. Their knowledge is based on initial energy ideas.
Summarizing the knowledge about energy dependencies revealed on the basis of experiments, it is necessary to highlight the most important of them - the relationship between the formation of new substances and the energy effect of the reaction, since energy changes, according to D.I. Mendeleev, represent the internal content of chemical reactions. It is important to bring students to a conclusion that complements the previous ones: the process of formation of new substances is associated with energy changes. Their important characteristic is the thermal effect of the reaction.
This knowledge is the basis for classification based on energy, dividing reactions into exo- and endothermic reactions.
Based on the electronic theory of the structure of matter, one of the most complex and information-intensive types of reactions is studied - redox ones. The most important concepts here are:
oxidation state;
oxidation processes / recovery;
oxidizing and reducing agent;
the actual redox reaction.
The formed concept of the redox reaction must be introduced into common system knowledge about the chemical process. The need for students to operate with the concept of “oxidation-reduction reaction” requires that they develop the ability to use chemical language. A generalized skill of students when studying redox reactions will be the ability to create equations for specific reactions.
When studying various classes of inorganic compounds and systematizing chemical elements, knowledge about redox reactions is supplemented, deepened and improved (familiarization with specific oxidizing agents and reducing agents occurs). A qualitatively new stage in the study of redox reactions will be the theory of electrolytes, in which the teacher introduces students to a new type of oxidizing and reducing agents - ions, and identifies and reveals the patterns of such reactions in aqueous solutions. When studying nitrogen and phosphorus, students' knowledge is replenished with new specific examples of oxidation and reduction. The reactions of nitric acid with metals are analyzed, and the skills of drawing up equations are improved. Next, electrolysis and corrosion of metals are studied as a type of redox processes.
Upon completion of student training, the general classification of chemical reactions should look like this:
Figure 2. Classification of chemical reactions.
2.3 Formation of knowledge about ion exchange reactions
Studying the theory of electrolytic dissociation allows us to deepen and expand knowledge about the reaction, differentiate the features of the course of exchange and redox reactions. Students acquire the ability to compose ionic and ion-electronic reaction equations and recognize electrolyte exchange reactions. Particular attention is paid to the problematic study of these reactions, mechanisms and patterns of their occurrence. The study of electrolyte reactions focuses on exchange reactions.
Ion exchange reactions are even more abstract compared to conventional molecular ones. As a result, the path to their knowledge should be as follows: a brief ionic equation, a complete ionic equation - an equation in molecular form - experiment.
Consider, for example, methods for developing knowledge about ion exchange reactions in the light of the theory of acid-base interactions.
Most ion exchange reactions in aqueous solutions can be considered in the light of ideas about acid-base interactions.
From the standpoint of the protolytic theory, acids are particles (ions, molecules) capable of donating a proton (proton donors), and bases are particles capable of attaching a proton (proton acceptors). For example, acetic acid CH 3 COOH in an aqueous solution donates protons to a base, the role of which is played by a water molecule. In this case, hydrozonium ions H 3 O + and a new base CH 3 COO - are formed. In such a system, a weak acid corresponds to a strong base CH 3 COO -. They are called conjugate acid and base, respectively. In a conjugate system, a strong acid corresponds to a weak base, and vice versa, a weak acid corresponds to a strong base. In such systems, different ions always compete with each other to bind a proton, for example in the system:
NO 2 - + HSO 4 - =HNO 2 + SO 4 2-.
The ions NO 2 - and SO 4 2- compete. Nitrite ions bind protons more strongly, since HNO 2 is more weak acid, than HSO 4 - .
To teach schoolchildren the ability to analyze the course of reactions, it is necessary to apply the empirical rules that are most understandable to them:
Exchange reactions in aqueous solutions proceed in the direction of the formation of a weak electrolyte, an insoluble or slightly soluble substance, or a gaseous product.
Strong acids displace weak acids from salt solutions. Heavier and less volatile acids displace less heavy and more volatile acids from salt solutions. The equilibrium in these cases is shifted towards the formation of a weaker or more volatile acid.
Strong bases displace weaker bases from salt solutions.
Strong electrolytes in dilute solutions have almost the same degree of dissociation and dissociate irreversibly. Medium and weak ones differ in the degree of dissociation and dissociate reversibly.
Ion exchange reactions in aqueous media are essentially reversible. A necessary condition for irreversibility is the removal of at least one of the reaction products. In the case when the composition of the starting substances and reaction products includes weak electrolytes, the exchange reactions are always reversible and we can only talk about a shift in equilibrium towards the weaker electrolyte.
To effectively consolidate the rules when analyzing ionic equations, you can invite students to use tables containing rows of acids, arranged in descending order of dissociation constant values (see appendix). Strong acids are shown as electrolytes of approximately equal strength. This table is used together with the corresponding exercises.
We can conditionally assume that the equilibrium of reactions in which the initial and resulting acids differ in ionization constants by at least one order of magnitude is practically shifted towards the weaker electrolyte. When solving problems, you can also use a displacement table of acids (see appendix), in which the formulas of acids in a row and column are arranged in descending order of dissociation constant. The direction of the arrow at the intersection of a row and a column indicates the acid being displaced or the equilibrium shifting towards the corresponding acid. Double arrows indicate the establishment of equilibrium at approximately equal acid concentrations. The proposed table can also be part of a set of reference materials for tests and exams.
2.4 Formation of knowledge about the kinetics of chemical reactions
Questions of the kinetics of chemical processes and chemical equilibrium are the most difficult not only for students, but also for teachers. When studying this material, a method based on students’ own cognitive activity is quite advantageous and promising. According to this method, the teacher does not explain new material, but organizes the cognitive activity of students, who observe observations, carry out calculations, model, find answers to questions posed by the teacher, and comprehend the results of their own activities. Properly organized cognitive activity leads schoolchildren to certain conclusions and the independent creation of knowledge.
All educational material is divided into 6 lessons:
The rate of a chemical reaction.
dependence of the rate of a chemical reaction on external factors.
The influence of temperature on the rate of a chemical reaction.
5-6. Chemical equilibrium and its displacement.
So, let's take a closer look at each stage of knowledge formation on this topic.
Lesson 1. Rate of a chemical reaction
The discussion of the new material begins with a demonstration of the following experiment: the interaction of hydrochloric acid with magnesium and iron. Students see that these two reactions proceed differently: with iron the reaction is much slower than with magnesium. Thus, the teacher leads students to the conclusion that chemical reactions can be characterized by certain rates.
Before students can come to an understanding of the rate of a chemical reaction, it is necessary to discuss the general "concept of rate." To do this, students are asked questions:
What is mechanical movement? (This is the length of the path traveled by the physical body per unit of time).
What changes over time as the film rolls? (The number of scrolled frames changes).
Each time the teacher emphasizes that the speed of a process is a change in some quantity per unit of time.
Now we need to find a quantity that changes over time during a chemical reaction. The teacher reminds that a chemical reaction occurs when particles collide. It is clear that the more often these collisions occur, the higher the reaction speed will be. Based on this, students are asked to formulate a definition of the rate of a chemical reaction. After listening to the assumptions, the teacher leads the students to a more precise definition: the rate of a chemical reaction is the number of collisions or the number of elementary reaction events per unit time. But the number of collisions cannot be calculated, so it is necessary to find another quantity that also changes over time during the course of a chemical reaction. The starting substances are converted into reaction products, which means the amount of the substance changes.
The change in any value is found as the difference between the initial and final values and is denoted by the Greek letter Δ (delta). Since the initial amount of the starting substance is greater than the final amount, then:
Δ n = n 1 – n 2.
To measure reaction rates, you need to calculate how the amount of a substance changes per unit time:
If a reaction occurs in a solution or gaseous environment, then when comparing the rates of different reactions, it is necessary to take into account not just the amount of substance, but the amount of substance per unit volume, that is molar concentration, which is calculated by the formula:
C =  and measured in mol/l.
and measured in mol/l.
So, the rate of a reaction in a solution is the change in the concentration of a substance per unit time:
∆C = C 1 – C 2;
The discussion begins again on the issue of measuring rate by changing the concentration of reaction products and deriving a rate formula for such a case. When deriving this formula, it turns out that it is identical to the previous one. Students then derive the unit of measurement for the rate of a chemical reaction from the formula: [W] = 
The teacher makes a general conclusion: the rate of a reaction is the change in the amount or concentration of starting substances or reaction products per unit time.
Next, the teacher teaches students how to calculate the speed in the experiment: to 10 ml. 0.1M solution of hydrochloric acid add the same volume of 0.1 M sodium thiosulfate solution. Using a metronome or stopwatch, we count the time from the start of pouring the solutions until the end of the reaction (turbidity), the speed is about 7 seconds. The rate can be determined by the concentration of one of the starting substances, and the final reaction should be considered equal to 0. Then we get:
W=  .
.
The question is then discussed: Does the reaction rate remain constant throughout the chemical process or does it change? In order for students to come to the correct conclusion, the teacher asks leading questions:
Does the amount of starting materials change during the reaction?
How does the number of particle collisions change with decreasing concentration?
Schoolchildren conclude that the rate of a chemical reaction decreases over time. To confirm this fact, students are offered the following task: for a reaction proceeding in accordance with the equation
C4H9OH + HCl = C4H9Cl + HOH
The concentration of one of the substances was determined experimentally at different time intervals.
How will the rate of this reaction change over time?
Students calculate the rate of a chemical reaction in the first time period, then in the second, and so on:
W 1 =  = 0.0023 mol/l with W 2 =
= 0.0023 mol/l with W 2 =  = 0.0019 mol/l s
= 0.0019 mol/l s
W 3 =  = 0.0014 mol/l s W 4 == 0.0009 mol/l s
= 0.0014 mol/l s W 4 == 0.0009 mol/l s
 Figure 3. Dependence of reaction speed on time.
Figure 3. Dependence of reaction speed on time.
Based on the calculated speed values, a graph of the reaction speed versus time is plotted. The use of such small quantities causes difficulty for students, so for ease of construction the speed is multiplied by 10 3.
It is important to draw students' attention to the fact that the speeds are averaged, and for more accurate calculations it is necessary to reduce the time interval. In this regard, points are placed in the middle of time periods.
Analyzing the graph. The teacher once again formulates the main conclusion of the lesson: over time, the rate of a chemical reaction decreases.
Lesson 2. Dependence of the rate of a chemical reaction on external factors
There is a check at the beginning of the lesson. homework similar to what was solved in the previous lesson. In parallel to this, it is discussed why the rate of a chemical reaction decreases over time (the amount of starting substances decreases, and if the reaction occurs in solution, then their concentrations). Reducing the amount of starting substances leads to the fact that particles collide with each other less often, and therefore the rate of the chemical reaction decreases. It turns out that the rate of a chemical reaction depends on the concentration of the starting substances.
This conclusion must be confirmed experimentally: consider the reaction between solutions of sodium thiosulfate of different concentrations and hydrochloric acid (0.1 M). We dilute the previously prepared solution of 0.1 M sodium thiosulfate: 2.5 ml in the first glass. Na 2 S 2 O 3 + 5 ml solution. water; in the second 5 ml. Na 2 S 2 O 3 + 2.5 ml solution. water; pour 7.5 ml into the third.
undiluted Na 2 S 2 O 3 solution.
During the experiment, one of the students assists the teacher. The metronome is started simultaneously with the addition of 2.5 ml to each glass. of hydrochloric acid. The moment of draining the solutions is considered zero, then the time from the beginning of the reaction to turbidity is counted. The assistant writes down on the board the reaction time in each glass.
1st glass – 23s.
2nd glass – 15s.
3rd glass – 7s.
Based on changes in the concentration of hydrochloric acid, we calculate the reaction rates and draw a graph:
 W 1 = 0.043 mol/l s W 2 = 0.067 mol/l s W 4 = 0.143 mol/l s
W 1 = 0.043 mol/l s W 2 = 0.067 mol/l s W 4 = 0.143 mol/l s
Rice. 4. Dependence of reaction rate on concentration. Drawing a graph takes time, but it gives you irreplaceable skills. scientific research
reacting substances. After this, the teacher asks the question: will concentration affect the rate of reaction of gaseous and solid substances? The concentration of a gas is proportional to pressure, so changing the pressure (and therefore the concentration) changes the rate of the reaction.
Solid substances do not fall under this dependence, since pressure does not have a significant effect on them (except for very large ones). Thus, students begin to realize that the rate of chemical processes can be controlled. The teacher should emphasize that this is especially important for chemical industries (those industries that are based on reactions that occur most quickly are the most profitable). At the same time, some reactions are undesirable and their speed must be slowed down (for example, metal corrosion processes). Therefore, it is so important to know what the speed of a chemical reaction depends on.
Next, we discuss how the nature of a substance (its composition, type, strength of bonds) affects the rate of a chemical reaction. Students are asked to consider an example: the interaction of oxygen and hydrogen occurs instantly, but the interaction of nitrogen and hydrogen occurs very slowly. The teacher provides the following data: to break bonds in nitrogen molecules, the energy required is 942 kJ/mol, and in oxygen molecules - 494 kJ/mol. Now students understand that stronger nitrogen molecules are more difficult to react and the rate of such a reaction is very low. That is, students are led to the conclusion that the rate of a chemical reaction depends on the nature of the reacting substances.
Then the influence of the state of aggregation of a substance on the reaction rate is discussed. Students independently carry out the reaction between PbNO 3 and KJ in crystalline form and in solution and conclude that the rate of the chemical reaction depends on the state of aggregation of the substance. It should be added that reactions between gaseous substances proceed even faster and are often accompanied by an explosion. Collisions between gas particles and in solution occur throughout the entire volume, and reactions involving solids occur only on the surface. Then how can you increase the rate of chemical reactions involving solids? The teacher suggests to the students that it is necessary to increase, that is, to crush the substance. Students explore the influence of this factor using the example of the interaction of a piece of marble with hydrochloric acid and marble chips with hydrochloric acid. The conclusion is formulated again: the reaction rate depends on the degree of grinding of the solid.
Lesson 3. The effect of temperature on the rate of reaction
The discussion of the new material begins with a demonstration of the interaction of 0.1 M solutions of sodium thiosulfate and hydrochloric acid. At room temperature and at a temperature 10˚C above room temperature.
To do this, the solutions are heated in a water bath with constant stirring. Experience shows that at room temperature the solution becomes cloudy after 11 seconds, and at elevated temperatures – after 5 seconds. Students independently calculate the speeds of both processes:  W 1 =
W 1 =  = 0.009 mol/l with W 2 =
= 0.009 mol/l with W 2 =
= 0.02mol/l s
γ =
 .
.
Thus, the rate of reaction is directly proportional to temperature.
Next, the students, together with the teacher, calculate how many times the reaction rate increased when the temperature increased by 10˚C The number γ is the temperature coefficient of the rate of a given reaction. The temperature coefficient shows how many times the reaction rate increases when the temperature increases by 10˚C. ּ To reinforce the concept of the temperature coefficient of reaction rate, students solve a series of tasks of increasing complexity. An example of a more complex level problem could be the following:
temperature coefficient
reaction rate is 3, how many times does the reaction rate increase when the temperature increases from 20 to 50˚C? To solve this problem, you can give a ready-made formula, but then students will not catch the essence. Therefore, it is better to derive the formula logically. Let us assume that the initial rate of the chemical reaction is 1 mol/l
s, i.e. at a temperature of 30˚C the reaction rate is equal to:
Now let's calculate the reaction rate at 40˚C
(W 3) and at 50˚С (W 4):
 ,
W 3 = W 2 γ = 9 mol/l s
,
W 3 = W 2 γ = 9 mol/l s
 W 4 = W 3 γ = 27 mol/l s
W 4 = W 3 γ = 27 mol/l s
This formula is a mathematical expression of van't Hoff's rule. You can tell students that the famous Dutch scientist J. Van't Hoff came to the conclusion that the rate of most reactions increases by 2-4 times with every 10˚C increase in temperature based on experimental studies.
W 2 = W 1 γ = 3 mol/l s
Now we need to understand why temperature affects the reaction rate. The teacher leads students to the idea that the energy imparted to a substance when heated is spent on destruction chemical bonds starting materials.
By demonstrating the following figure, the teacher shows how the electron density of chemical bonds changes when iodine interacts with hydrogen:
Rice. 5 Scheme of PAA formation using the example of the interaction of iodine and hydrogen.
When molecules collide, an electron cloud common to 4 atoms is formed. It is unstable: the electron density from the region between the atoms of the starting substances seems to flow into the region between the iodine and hydrogen atoms.
Such an intermediate compound formed by two molecules is called an intermediate activated complex (PAC). It exists for a short time and breaks down into two molecules (in this case HJ).
For the formation of PAA, energy is required to destroy the chemical bonds inside the colliding molecules. This energy is called activation energy.
Activation energy is the energy required for 1 mole particles to form an activated complex.  G
G
Graphically this process looks like this:
Thus, the activation energy is the energy barrier that the starting substances must overcome in order to turn into reaction products: the lower the activation energy, the higher the rate of the chemical reaction.
Summing up the lesson, the teacher formulates a conclusion: when heated, the rate of a chemical reaction increases because the number of molecules capable of overcoming the energy barrier increases.
The concept of “catalysis” is also formed on the basis of experiment. Students are shown a bottle of hydrogen peroxide. They see that there are no signs of the reaction progressing. But students know that hydrogen peroxide decomposes over time. Then the teacher asks: how can one speed up the decomposition process. Most likely, answers will follow about increasing the temperature to the point at which decomposition will be noticeable. The teacher demonstrates the experience of heating hydrogen peroxide. When a smoldering splinter is presented, students see that it goes out (which means the oxygen released is clearly not enough to maintain combustion). That is, heating slightly increases the rate of a chemical reaction. Then the teacher adds manganese dioxide MnO 2 to the bottle with hydrogen peroxide.
Even without a smoldering splinter, students observe the instantaneous release of gas.
Then, instead of MnO 2, the teacher adds cobalt (II) oxide CoO (the reaction proceeds even more violently), and then carries out the same experiment with CuO (in this case the reaction proceeds very slowly).
The teacher reports that substances that can increase the rate of a chemical reaction are called catalysts.
Through experience, schoolchildren were convinced that not every substance can be a catalyst and accelerate a chemical process. Hence the conclusion is that the action of catalysts is selective.
Then the teacher draws the students' attention to the fact that the substances that accelerated the reaction were not consumed themselves. If you filter them and dry them, it turns out that their mass has not changed. To explain this fact, the teacher schematically shows the process of a catalytic reaction:
Stage 1. A + K = AK
Stage 2. AK + B = AB + K.
Thus, substance K remains quantitatively unchanged.
Now it is necessary to understand the reason why catalysts increase the rate of chemical reactions. The increase in the reaction rate under the influence of a catalyst is explained by the fact that each of the two stages with a catalyst has a lower energy barrier compared to the direct reaction of the initial substances.
Lesson 5-6. Chemical equilibrium and its displacement The lesson begins with updating the knowledge acquired in previous lessons, in particular about the energy barrier and the formation of PAK. Moving on to
new topic

, the teacher finds out what PAA turns into: reaction products or starting substances.
The transformation of starting substances into reaction products is called a forward reaction, and the transformation of products into starting substances is called a reverse reaction. The teacher tells students that the reaction of iodine with hydrogen, taken as an example, is a reversible process, and in fact, most reactions are reversible.
Next, students are informed that over time, the rate of the forward reaction decreases, and the rate of the reverse reaction first equals 0 and then increases. To illustrate this more clearly, the teacher shows students a graph, which they transfer to their notebooks.
Analyzing the graph, students come to the conclusion that at some point in time the speeds of the forward and reverse reactions equalize. This fact indicates the onset of equilibrium.
Students are asked the question: do both reactions stop when chemical equilibrium occurs?
If the reactions stop, then if the conditions affecting the rate of the forward or reverse reaction change, nothing will happen.
To test this fact, students are shown the following experiment: two test tubes, closed with stoppers and connected by a glass tube, are filled with nitrogen dioxide. NO 2 dimerizes when cooled, and when heated, the reverse reaction occurs:
NO 2 (brown) N 2 O 4 (colorless)
Place one test tube in hot water, the other in a glass with pieces of ice.
Upon cooling, dimerization increases and the color of the mixture becomes less intense. When heated, N 2 O 4 decomposes and the color of the mixture intensifies. A change in gas color when conditions change indicates that reactions continue to occur. If you remove the test tubes from the beaker, after some time the color in them will even out.
The teacher asks you to think about what factors influence the shift in balance. Students' responses highlight concentration, temperature, and pressure.
Moreover, they had already observed the effect of temperature in an experiment with nitric oxide.
The study of the effect of concentration is carried out in the experiment of the interaction of potassium thiocyanate with iron (III) chloride:
KCNS + FeCl 3 = Fe(CNS) 3 + KCl By increasing the concentration of the starting substances, the color of the solution becomes more intense, and when KCl is added to the reacted solution, the color becomes less saturated. Thus, students see that an increase in the concentration of starting substances leads to a greater formation of reaction products (an increase in the rate of the forward reaction), and therefore to a shift in equilibrium to the right and vice versa.
Students are no longer studying the influence of the next factor - pressure - not experimentally, but by modeling the reaction process. Students already know that pressure primarily affects reactions between gases. The teacher formulates
general principle
Le Chatelier: if a system in equilibrium is affected by changing concentration, pressure, temperature, then the equilibrium will shift in the direction of the reaction that will reduce this effect.
general principle
The effect of pressure is usually considered using the example of ammonia synthesis reaction:
N2 + 3H2 = 2NH3.
Students are reminded of the relationship between pressure and temperature. Since the dependence is directly proportional, an increase in pressure, and therefore the volume of the initial gas components, shifts the equilibrium towards the formation of ammonia (towards a decrease in volume). The issue of shifting equilibrium under conditions of decreased pressure is also discussed. Schematically, both conclusions can be written as follows: Decrease p.
Increase in river .
The teacher formulates the conclusion: an increase in pressure causes a shift in equilibrium towards the reaction that leads to the formation of fewer gases, therefore, to a decrease in pressure. A decrease in pressure causes a shift in equilibrium towards the reaction that leads to the formation
more
By independently analyzing this equation, students realize that while the forward reaction is endothermic, the reverse reaction is exothermic. Students may have difficulty completing these reactions, so the teacher can ask guiding questions about how the temperature of a system changes when heat is absorbed (decreases) and how it changes when heat is released (increases). Having come to such conclusions, students themselves formulate the conclusion: when the temperature increases, equilibrium shifts towards the endothermic (direct), and when it decreases, towards the exothermic (in this case, reverse).
The completeness of the proposed material in this method corresponds to educational standards. This method allows you to activate students' thinking.
Conclusion
In conclusion, I would like to once again note those methods and techniques that are used in the formation of the main sections of the concept of a chemical reaction.
the main role When studying each component of the concept of “chemical reaction”, it is assigned to a chemical experiment. It most clearly reflects external signs and phenomena occurring during interaction, and also reflects the influence of external factors on the reacting substances. He solves diverse problems of education (labor, cultural, ethical, worldview, environmental); development (memory, thinking, imagination, creative independence); training. In the learning process, it serves as a source of knowledge, performs the function of a method (knowing chemical objects, testing educational hypotheses, solving educational problems ), as well as the function of a teaching tool (evidence of the truth of judgments, illustration, application of knowledge and skills), a means of education and development of students. When studying many topics, chemical experiment is used in parallel with modeling: writing chemical formulas of substances, compiling process models from them, drawing graphic illustrations of processes. there are specific substances (it is not the formula that reacts, but the substance). In this regard, the interpretation of reaction equations must be correct. For example, in the reaction: 2H 2 + O 2 = 2H 2 O, the formulation of the process should be as follows: 2 moles of hydrogen react with 1 mole of oxygen and 2 moles of water are formed (and not two al-two plus o-two equals two al-two-o).
The use of various outline diagrams makes it easier for students to memorize voluminous material. For example, using the diagram “The rate of a chemical reaction and its dependence on various factors” (see appendix) helps to assimilate, remember and reproduce accumulated knowledge on this topic. Such schemes can consist of several blocks and are compiled step by step as each block is studied.
When studying various classes of simple and complex compounds, the teacher can use mineral collections. So, for example, when studying the topic “Sulfur and its compounds,” it is necessary to familiarize students with the mineral itself in order to study it physical properties, which also allows one to overcome the formalism of knowledge. In addition, for the same purpose, conduct an excursion for students, during which they can observe the formation of a sulfur film on puddles, stones, and grass after rain near hydrogen sulfide sources. Using the example of sulfur-containing minerals (sulfates, sulfides), students can supplement their knowledge of redox processes occurring in nature.
Particular attention is paid to methods that allow students to intensify independent activity. It is known that the time of beginning to study chemistry at school (8th grade) corresponds to the teenage period of personality development of students (11-12 – 14-15 years). At this age, shapes become the most attractive for a teenager. conducting classes
allowing them to show independence and initiative. He learns ways of acting more easily when the teacher only helps him. Examples of activities that actively use this principle are discussed in more detail in the paragraphs “Introduction of the concept of a chemical reaction”, “Formation of knowledge about the kinetics of chemical reactions”.
So, in the considered methodological approaches the following methods are used:
general logical: abstraction, inductive approach to deriving concepts, generalization, concretization and others.
general pedagogical: story, reasoning, conversation and others.
These methods are used in combination, since often the use of any one group of methods does not lead to effective positive results. The integration of these methods in a certain combination leads to the emergence of a method of teaching chemistry.
Interest in academic subject largely depends on the form in which the teacher presents the material being studied and how captivatingly and intelligibly he explains it. It is these qualities that must be taken into account when choosing teaching methods, because only a correctly chosen method will activate interest in learning and enhance the motivation to learn.
Bibliography
Kuznetsova L. M., Dronova N. Yu., Evstigneeva T. A. On the methodology for studying chemical kinetics and chemical equilibrium // Chemistry at school. – 2001. – No. 9. – p.7.
Kuznetsova N. E. Methods of teaching chemistry: Textbook. manual for pedagogical students. Institute of Chemistry. and biol.
specialist. – M.: Education, 1984. –415 p., ill.
Kuznetsova N. E. Formation of systems of concepts in teaching chemistry. – M.: Education, 1989. – 144 p. Mukhina V.S. Age-related psychology
: phenomenology of development, childhood, adolescence: Textbook for students. universities – 9th ed., stereotype. –M.: Publishing Center “Academy”, 2004. – 456 p.
Pak M. S. Fundamentals of didactics of chemistry: textbook. – St. Petersburg: Publishing house of the Russian State Pedagogical University named after. A. I. Herzen, 2004. –307 p.
Stabaldina S. T. Principles and laws of dialectics in teaching chemistry // Chemistry at school. – 2003. – No. 7. – p.16.
Trofimova I. V. Ion exchange reactions in aqueous solutions // Chemistry at school. – 2005. – No. 10. – pp. 10-16.
Turlakova E. V. Use of outline diagrams in studying the patterns of chemical reactions.
// Chemistry at school. – 1997. – No. 1. – p.6.
Chemistry. 8th grade: Lesson plans (based on the textbook by L. S. Guzei and others). I half of the year / Author. – comp. S. Yu. Diblenko.
– Volgograd: Teacher, 2004. – 144 p.
Chemistry. 8th grade: Lesson plans (based on the textbook by L. S. Guzei and others). II half of the year / Author. – comp. S. Yu. Diblenko. – Volgograd: Teacher, 2004. – 168 p. Chemistry. Grade 9: Lesson plans (based on the textbook by L. S. Guzei and others). I half of the year / Author. – comp. S. Yu. Diblenko, E. A. Smirnova, S. M. Kolmykova. – Volgograd: Teacher, 2005. – 169 p.
Shelinsky G.I. Urgent issues of formation of the most important chemical concepts of chemistry in initial stage training // Chemistry at school. – 2001. – No. 5. – p.17.
Shilov V.I. Use of minerals in the formation of chemical concepts // Chemistry at school. – 2006. – No. 3. – p.32.
Application
|
Series of acids |
Order of the dissociation constant |
|
1. NSJ 4 , HI, HBr, HCI,NMP0 4 , H 2 S0 4 , H 2 Se0 4, H 2 Cr 2 0 7 , HN0 3 |
|
|
2. N 4 R 2 0 7 2 Cr0 4 = nude 3 = НВг0 3 ≈ H 2 S 2 0 3 |
10 -1 |
|
3. NSg 2 ABOUT 7 - = NSJ 2 = HSe0 4 - ≈ H 2 S0 3 = NSABOUT - 4 ≈ H.S. 2 0 3 4 = N 3 P0 3 |
10 -2 |
|
4. N 2 Te =H 2 Se0 3 = N 2 Te0 3 H 3 As0 4 3 P0 4 = N 3 R 2 ABOUT 4 |
10 -3 |
|
5. H 2 Se 2 IN 4 0 7 HF= NN0 2 |
10 -4 |
|
6. CH 3 UNS |
10 -5 |
|
7. N 2 P0 3 = H 2 As0 4 4 = N 2 C0 3 |
10 -6 |
|
8. NTeOz 6 Te0 6 = NSJ =H 2 S = H.S.0 3 = N 2 P0 4 |
10 -8 |
|
9. NWYU =HSe0 3 - |
10 -9 |
|
10.H 2 Si0 3 =H 4 Si0 4 3 As0 3 =H 3 B0 3 + |
10 -10 |
|
11. HSe"= nude 4 |
10 -11 |
|
12. H 3 Si0 4 N 2 0 2 = HAsABOUT 2- |
10 -12 |
|
13. H.S. - = NAYU 2 = NTe - ≈ HPO 4 2- |
10 -13 |
|
14 H 2 SiO 4 2- |
10 -14 |
|
15. N 2 ABOUT |
10 -16 |
|
HClO 4 |
HMnO 4 |
H 2 SO 4 |
HNO 3 |
H 2 CrO 4 |
H 2 SO 3 |
HSO 4 - |
H 3 P.O. 4 |
HNO 2 |
HCrO 4 |
H 2 CO 3 |
H 2 S |
HSO 3 |
H 2 P.O. 4 |
H 2 SiO 3 |
N.H. 4 + |
HCO 3 - |
H.S. - |
HPO 4 2- |
|||||
|
HClO 4 |
|||||||||||||||||||||||
|
H.M. 4 |
|||||||||||||||||||||||
|
H 2 SO 4 |
|||||||||||||||||||||||
|
HNO 3 |
|||||||||||||||||||||||
|
H 2 C 4 |
|||||||||||||||||||||||
|
H 2 O 3 |
|||||||||||||||||||||||
|
HSO 4 - |
|||||||||||||||||||||||
|
H 3 P.O. 4 |
|||||||||||||||||||||||
|
HNO 2 |
|||||||||||||||||||||||
|
HCr - |
|||||||||||||||||||||||
|
H 2 CO 3 |
|||||||||||||||||||||||
|
H 2 S |
|||||||||||||||||||||||
|
HSO 3 - |
|||||||||||||||||||||||
|
H 2 P.O. 4 - |
|||||||||||||||||||||||
|
H 2 SiO 3 |
|||||||||||||||||||||||
|
N.H. 4 + |
|||||||||||||||||||||||
|
HCO 3 - |
|||||||||||||||||||||||
|
H.S. - |
|||||||||||||||||||||||
|
HPO 4 2- |
approaches To formation and strengthening in children... volume knowledge the student receives... speed, accuracy of oculomotor reactions, ability to... chemical substances on taste buds causes formation ...Formation competitive advantages of industrial enterprises using the example of OJSC RudgormashCoursework >> ManagementResearched methodological approaches To formation And... reactions on their own strategic actions; - assess their competencies and abilities according to formation ... knowledge, essential for formation... transport, construction, chemical and petrochemical and... Development of the current annual plan for the current chemical production enterpriseCoursework >> ManagementAnd control in chemical industry and environmental management Department... 48 7.1. Methodical approaches To formation selling prices... and consolidation of received knowledge in the course of studying... industrial organic synthesis in reactions: ● dehydration (receiving... Chemical, physical environmental factors, measures to prevent harmful effects on the bodyTest >> EcologyAuditory, olfactory reactions, deterioration... petrochemical and chemical industry is allocated to... improvement remains methodological approaches to the study... of man. Knowledge listed above... 1) the main factor formation natural and artificial... |
TOPIC 6. PSYCHOPHYSIOLOGY OF ATTENTION
- 6.1. Approximate reaction
- 6.2. Neurophysiological mechanisms of attention
- 6.3. Methods for studying and diagnosing attention
In psychology, attention is defined as the process and state of tuning a subject to perceive priority information and perform assigned tasks.
The direction and concentration of mental activity during attention ensures more effective perception of information. In general terms, there are two main types of attention: involuntary and voluntary (selective, selective). Both types of attention have different functions, are formed differently in ontogenesis, and are based on different physiological mechanisms.
Estimated reaction (OR) was first described by I.P. Pavlov as the motor reaction of an animal to a new, suddenly appearing stimulus. It included turning the head and eyes towards the stimulus and was necessarily accompanied by inhibition of the current conditioned reflex activity. Another feature of OR was the extinction of all its behavioral manifestations upon repetition of the stimulus. The extinct OR was easily restored at the slightest change in the situation (see Reader 6.2).
Physiological indicators of RR. The use of polygraphic registration showed that OR causes not only behavioral manifestations, but also a whole range of vegetative changes. Reflection of these generalized changes are various components of the OR: motor (muscular), cardiac, respiratory, galvanic skin, vascular, pupillary, sensory and electroencephalographic (see topic 2). As a rule, when a new stimulus is presented, the muscle tone, the frequency of breathing and pulse changes, the electrical activity of the skin increases, the pupils dilate, and sensory thresholds decrease. In the electroencephalogram, at the beginning of the indicative reaction, generalized activation occurs, which manifests itself in blockade (suppression) alpha rhythm and its replacement by high-frequency activity. At the same time, the possibility arises of unification and synchronous operation of nerve cells not according to the principle of their spatial proximity, but according to the functional principle. Thanks to all these changes, a special state of mobilization readiness of the body arises.
More often than others, in experiments aimed at studying OR, indicators of galvanic skin response are used ( GSR). It is particularly sensitive to the novelty of the stimulus and is modally nonspecific, i.e. does not depend on what particular stimulus causes the OR. In addition, GSR decays quickly, even if OR is caused by a painful stimulus. However, GSR is closely related to the emotional sphere, so the use of GSR in the study of OR requires a clear separation of the actual indicative and emotional components of the response to a new stimulus.
Neural model of the stimulus. The mechanism of emergence and extinction of OR was interpreted in the concept of a neural stimulus model proposed by E.N. Sokolov. According to this concept, as a result of repetition of a stimulus, a “model” is formed in the nervous system, a certain trace configuration in which all parameters of the stimulus are recorded. Approximate reaction occurs in cases where a mismatch is detected between the current stimulus and the generated trace, i.e. "nervous model" If the current stimulus and the neural trace left by the previous stimulus are identical, then OR does not occur. If they do not coincide, then an indicative reaction arises and, to a certain extent, is stronger, the more different the previous and new stimuli are. Since the OR arises as a result of a mismatch of afferent stimulation with the “nervous model” of the expected stimulus, it is obvious that the OR will last as long as this difference exists.
In accordance with this concept, the OR should be recorded for any noticeable discrepancy between two sequentially presented stimuli. There are, however, numerous facts that indicate that OR does not always necessarily arise when the stimulus parameters change.
Significance of the stimulus. The orientation reflex is associated with the body’s adaptation to changing environmental conditions, therefore the “law of force” is valid for it. In other words, the more the stimulus changes (for example, its intensity or degree of novelty), the greater the response. However, no less, and often a greater reaction can be caused by insignificant changes in the situation if they are directly addressed to the basic needs of a person.
It seems that a more significant and, therefore, somewhat familiar stimulus should, other things being equal, cause a smaller RR than a completely new one. The facts, however, tell a different story. The significance of the stimulus is often decisive for the occurrence of OR. A highly significant stimulus can evoke a powerful orienting response with little physical intensity.
- According to some ideas, the factors that provoke OR can be ordered into 4 levels, or registers:
- stimulus register;
- novelty register;
- intensity register;
- significance register.
Almost all stimuli pass the first level of assessment; the second and third registers work in parallel. Having passed through any of these two registers, the stimulus enters the last one and its significance is assessed there. Only after this final act of evaluation does the entire complex of the orienting reaction develop.
Thus, OR does not arise in response to any new stimulus, but only in response to one that is previously assessed as biologically significant. Otherwise, we would experience OR every second, since new stimuli act on us constantly. When assessing OR, therefore, one must take into account not the formal amount of information contained in the stimulus, but the amount of semantic, meaningful information.
Another important thing is that the perception of a significant stimulus is often accompanied by the formation of a response adequate reactions. The presence of motor components indicates that the OR represents a unity of perceptive and executive mechanisms. Thus, the OR, traditionally considered as a reaction to a new stimulus, represents special case indicative activity, which is understood as the organization of new types of activity, the formation of activity in changed environmental conditions (see Reader. 6.1).
REACTION MECHANISM. The concept is used in two basic senses. For complex reactions consisting of several stages, the REACTION MECHANISM is a set of stages as a result of which the starting substances are converted into products. For a simple reaction (elementary reaction, elementary stage), which cannot be decomposed into simpler chemical acts, clarify the MECHANISM OF THE REACTION. means identifying the physical processes that make up the essence of chemical transformation. For one particle (molecule in the ground or excited state, ion, radical, diffusion pair, singlet or triplet radical pair, complex) or two (rarely three) particles (molecules, ions, radicals, radical ions, etc. ), located in certain quantum states, changes in positions atomic nuclei
Hypothetical ideas regarding REACTION MECHANISM. are formed on the basis of available experiments. facts and theoretical results. analysis. New data may lead to changes or refinements of the proposed REACTION MECHANISM, bringing it ever closer to the true one.
Complex reactions. Stoichiometric
the equation, as a rule, does not reflect the true REACTION MECHANISM. Thus, the gas-phase thermally activated unbranched chain reaction H 2 + Br 2 2HBr consists of the following simple stages: thermodynamic initiation of Br 2; chain continuation + H 2 HBr + ; + + Br 2 HBr + ; + НВr Н 2 +; open circuit + + Br 2. The rate of the process is described by a complex equation, including the rate constants of all simple stages and the concentrations of the substances Br 2, H 2 and HBr.
Establishing the mechanism of a complex reaction begins with studying changes in time in the concentrations of starting substances and, if possible, intermediate substances, determining reaction orders for individual reagents under a wide range of varying conditions (temperature, initial partial and total pressures for gas-phase reactions; initial and total concentrations of reagents, nature of the solvent for reactions in solutions). Based on the data obtained, one or more possible reaction schemes are proposed and differential systems are drawn up.
equations. When solving these systems using a computer, a distinction is made between direct and inverse problems. In the direct problem, the rate constants and equilibrium constants are separated. simple stages, obtained experimentally or evaluated independently, are set by a computer, which numerically or graphically presents the results of solving a system of equations in the form of kinetic curves of a complex reaction. These curves are then compared with experiments. data.. To obtain the rate constants of those stages of a complex reaction in which highly active particles participate, it is informative to model these stages under special (“pure”) conditions, for example, by carrying out reactions at low temperatures (up to 100-70 K), in the ion source of a high-speed mass spectrometer pressure, in the cell of an ion cyclotron resonance spectrometer, etc. When studying heterogeneous catalytic reactions, it is important to independently study the adsorption of all substances participating in the reaction on the surface of the catalyst and study the spectra of the adsorbir. particles in the optical and radio frequency ranges, as well as establishing their nature by physical and physical-chemical methods (X-ray and UV photoelectron spectroscopy, Auger spectroscopy, electron energy loss spectroscopy, etc.).
Elementary reactions. To establish the REACTION MECHANISM p. attracted as a theoretical methods (see Quantum chemistry, Dynamics of an elementary act), and numerous experiments. methods. For gas-phase reactions, these are the molecular beam method, high-pressure mass spectrometry, mass spectrometry with chemical ionization, ion photodissociation, ion-cyclotron resonance, afterglow method in a flow, laser spectroscopy - selective excitation of individual bonds or atomic groups of a molecule, in including laser-induced fluorescence, intracavity laser spectroscopy, active coherent scattering spectroscopy. To study the REACTION MECHANISM. in condenser environments use methods: EPR, NMR, nuclear quadrupole resonance, chemical polarization of nuclei, gamma resonance spectroscopy, X-ray and photoelectron spectroscopy, reactions with isotope indicators (labeled atoms) and optically active compounds, carrying out reactions at low temperatures and, spectroscopy (UV, IR and Raman scattering), chemiluminescent methods, polarography, kinetic methods for studying fast and ultrafast reactions (pulse photolysis, methods of continuous and stopped jet, temperature jump, pressure jump, etc.). Using these methods, knowing the nature and structure of the initial and final particles, it is possible to establish with a certain degree of certainty the structure of the transition state (see Activated complex theory), find out how the initial molecule is deformed or how the initial particles, if there are several of them, come together (changes in interatomic distances, angles between bonds), how the polarizability of chemical bonds changes, whether ionic, free radical, triplet, or others are formed. active forms whether the electronic states of molecules, atoms, and ions change during the reaction.
For example, quantum chemical calculations indicate that during the bimolecular reaction between HNCO and CH 3 OH, as the distance between the C atom of the -NCO group and the O atom of the alcohol decreases from 30 to 10 nm, the charges q N and q O on the N and O atoms change group -N=C=O and the occupancy of bonds P N=C and P C=0.
A sharper rate of change in charge on N (Dq N = 0.47) compared to the change in charge on O (Dq O = 0.18), as well as a decrease in the population of the N=C bond (DP N=C = 0.58) in comparison with the C=O bond (DP C =O = 0.35) allows us to conclude that the hydroxyl CH 3 OH preferentially attaches to the N=C bond with the formation of the urethane group -NHC(O)OCH 3 . IN simple cases Quantum chemistry methods make it possible to calculate the potential energy surface (PES) along which the reaction occurs. In more difficult cases

It is possible to establish only one of the PES profiles, which displays the type of reaction coordinate. Modern calculations and experiments. methods make it possible to establish a more complex course of elementary reactions than was previously imagined. For example, reactions of the type, where X - F or I, can occur with the participation of different electronic states of particles:

When studying elementary reactions of even the simplest particles using the mol. beams, the presence of several reaction channels with their own enthalpies DH 0 and cross sections is revealed:

Investigating the intensity pattern of angular scattering of products in mol. beams, you can get a direct microscopic peak.
![]()
information about the details of the pier. interactions. For example, the K + I 2 reaction proceeds according to the disruption mechanism, when each K atom incident on the I 2 molecule picks up one I atom, moving in the forward direction, without having a strong effect on the second atom I. In the limiting case of such a REACTION MECHANISM p.

atom I acts as an “observer”, since its momentum after the reaction event remains the same as before it (MR type “observer-disruption”). However, the behavior of the KI product in the K + CH 3 I reaction differs significantly from that described for the K + I 2 reaction: the K + CH 3 I reaction is carried out with partner particles approaching so closely that the KI product should “ricochet”, as if solids were colliding balls (mechanism of measurement and cocheting). The approach of a flying K atom to a CH 3 I molecule is most effective in the K...I-CH 3 configuration, i.e. from the iodine end of the molecule (“orientation effect of the target molecule”). For the reaction between an alkali metal atom M and a halogen molecule X 2, the so-called harpoon mechanism is postulated, in which an electron jumps from the M atom to the X 2 molecule with the formation of particles M + and X - 2, which, moving rapidly towards each other, interact with the formation of a vibrationally excited product M + X - . Often a bimolecular reaction occurs in two "microscopic" reactions. stage with preliminary formation of intermediate complex:
In a detailed analysis, the REACTION MECHANISM p.

Sometimes there is a need to explicitly consider acts of energy transfer between molecules or from the same energy. levels of the molecule to others. This is especially evident in gas-phase reactions. For example, the monomolecular reaction AB A + B can only occur if the AB molecule has internal. energy greater than the activation energy of the reaction. Such active molecules AB* are formed as a result of inelastic collisions of AB with surrounding molecules X (thermodynamic activation), as well as during irradiation with light or electron impact.
A thermodynamically elementary reaction, along with the chemical transformation itself (rate constant k*), should include acts of activation and deactivation (rate constants k a and k d): Due to the increase in the concentration of X with increasing pressure, this reaction is second order at low pressures and first order at high pressures (see Monomolecular reactions). Strictly speaking, each of the above reactions must be described by a system of kinetic equations corresponding to the microscope. acts involving particles with different energy populations. levels. The transfer of energy from vibrational to electronic levels of a molecule is an important stage, for example, during the interaction in the ground electronic state of 2 P 3/2 with a vibrationally excited HCl molecule (vibrational
quantum number
u=1):