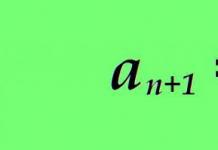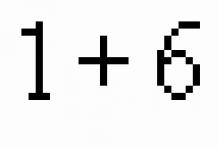Definition 1
Molecular Kinetic Theory- this is the doctrine of the structure and properties of matter, based on the idea of the existence of atoms and molecules, as the smallest particles of chemical substances.
The main provisions of the molecular kinetic theory molecules:
- All substances can be in liquid, solid and gaseous state. They are formed from particles that are made up of atoms. Elementary molecules can have a complex structure, that is, they can contain several atoms. Molecules and atoms are electrically neutral particles that, under certain conditions, acquire an additional electric charge and transform into positive or negative ions.
- Atoms and molecules move continuously.
- Particles with an electrical nature of force interact with each other.
The main provisions of the MKT and their examples have been listed above. Between particles there is a small gravitational influence.
Figure 3. one . one . The trajectory of a Brownian particle.
Definition 2
The Brownian motion of molecules and atoms confirms the existence of the main provisions of the molecular kinetic theory and substantiates it experimentally. This thermal movement of particles occurs with molecules suspended in a liquid or gas.
Experimental substantiation of the main provisions of the molecular kinetic theory
In 1827, R. Brown discovered this movement, which was due to random impacts and movements of molecules. Since the process was chaotic, the blows could not balance each other. Hence the conclusion that the speed of a Brownian particle cannot be constant, it is constantly changing, and the movement of the direction is depicted as a zigzag, shown in Figure 3. one . one .
A. Einstein spoke about Brownian motion in 1905. His theory was confirmed in the experiments of J. Perrin in 1908 - 1911.
Definition 3
Consequence from Einstein's theory: offset square< r 2 >Brownian particle relative to the initial position, averaged over many Brownian particles, is proportional to the observation time t .
Expression< r 2 >= D t explains the diffusion law. According to the theory, we have that D increases monotonically with increasing temperature. Random motion is visible in the presence of diffusion.
Definition 4
Diffusion- this is the definition of the phenomenon of penetration of two or more contiguous substances into each other.
This process occurs rapidly in an inhomogeneous gas. Thanks to diffusion examples with different densities, a homogeneous mixture can be obtained. When oxygen O 2 and hydrogen H 2 are in the same vessel with a partition, when it is removed, the gases begin to mix, forming a dangerous mixture. The process is possible when hydrogen is at the top and oxygen is at the bottom.
Interpenetration processes also occur in liquids, but much more slowly. If we dissolve a solid, sugar, in water, we get a homogeneous solution, which is a clear example of diffusion processes in liquids. Under real conditions, mixing in liquids and gases is masked by rapid mixing processes, for example, when convection currents occur.
Diffusion solids distinguished by its slow speed. If the interaction surface of metals is cleaned, then it can be seen that over a long period of time, atoms of another metal will appear in each of them.
Definition 5
Diffusion and Brownian motion are considered related phenomena.
With the interpenetration of particles of both substances, the movement is random, that is, there is a chaotic thermal movement of molecules.
The forces acting between two molecules depend on the distance between them. Molecules have both positive and negative charges. At large distances, the forces of intermolecular attraction predominate, and at small distances, repulsive forces predominate.
Picture 3 . 1 . 2 shows the dependence of the resulting force F and the potential energy E p of the interaction between molecules on the distance between their centers. At a distance r = r 0, the interaction force vanishes. Given distance is conventionally taken as the diameter of the molecule. At r = r 0 the potential energy of interaction is minimal.
Definition 6
To move two molecules apart with distance r 0 , E 0 should be reported, called binding energy or potential well depth.

Figure 3. one . 2.The power of interaction F and potential energy of interaction E p two molecules. F > 0- repulsive force F< 0 - force of gravity.
Since the molecules are small, simple monatomic ones can be no more than 10 - 10 m. Complex ones can reach sizes hundreds of times larger.
Definition 7
The random random movement of molecules is called thermal movement.
As the temperature rises, the kinetic energy thermal movement. At low temperatures, the average kinetic energy, in most cases, turns out to be less value depth of the potential well E 0 . This case shows that the molecules flow into a liquid or solid with an average distance between them r 0 . If the temperature rises, then the average kinetic energy of the molecule exceeds E 0, then they fly apart and form a gaseous substance.
In solids, molecules move randomly around fixed centers, that is, equilibrium positions. In space, it can be distributed in an irregular manner (in amorphous bodies) or with the formation of ordered bulk structures (crystalline bodies).
Aggregate states of substances
The freedom of thermal motion of molecules is seen in liquids, since they do not have binding to centers, which allows movement throughout the volume. This explains its fluidity.
Definition 8
If the molecules are close, they can form ordered structures with several molecules. This phenomenon has been named close order. distant order characteristic of crystalline bodies.
The distance in gases between molecules is much greater, therefore active forces are small, and their motions go along a straight line, waiting for the next collision. The value 10 - 8 m is the average distance between air molecules in normal conditions. Since the interaction of forces is weak, the gases expand and can fill any volume of the vessel. When their interaction tends to zero, then one speaks of a representation ideal gas.
Kinetic model of an ideal gas
In microns, the amount of matter is considered proportional to the number of particles.
Definition 9
mole- this is the amount of a substance containing as many particles (molecules) as there are atoms in 0, 012 to g of carbon C 12. A carbon molecule is made up of one atom. It follows that 1 mole of a substance has the same number of molecules. This number is called constant Avogadro N A: N A \u003d 6, 02 ċ 1023 mol - 1.
Formula for determining the amount of a substance ν is written as the ratio N of the number of particles to the Avogadro constant N A: ν = N N A .
Definition 10
The mass of one mole of a substance call the molar mass M. It is fixed in the form of the formula M \u003d N A ċ m 0.
Expression molar mass produced in kilograms per mole (kg/m o l b).
Definition 11
If the substance has one atom in its composition, then it is possible to speak of atomic mass particles. The unit of an atom is 1 12 masses of the carbon isotope C 12, called atomic mass unit and written as ( a. eat.): 1 a. e. m. \u003d 1, 66 ċ 10 - 27 to g.
This value coincides with the mass of the proton and neutron.
Definition 12
The ratio of the mass of an atom or molecule of a given substance to 1 12 of the mass of a carbon atom is called relative weight.
If you notice a mistake in the text, please highlight it and press Ctrl+Enter
Matter is made up of particles.
Molecule is the smallest particle of a substance that has its basic chemical properties.
A molecule is made up of atoms. Atom- the smallest particle of a substance that does not divide in chemical reactions.
Many molecules are made up of two or more atoms held together. chemical bonds. Some molecules are made up of hundreds of thousands of atoms.
The second position of the molecular kinetic theory
Molecules are in continuous chaotic motion. This movement does not depend on external influences. The movement occurs in an unpredictable direction due to the collision of molecules. The proof is Brownian motion particles (discovered by R. Brown in 1827). Particles are placed in a liquid or gas and their unpredictable movement is observed due to collisions with the molecules of the substance.

Brownian motion
The proof of chaotic motion is diffusion- the penetration of molecules of one substance into the gaps between the molecules of another substance. For example, we feel the smell of an air freshener not only in the place where it was sprayed, but it gradually mixes with air molecules throughout the room.
Aggregate state of matter
AT gases the average distance between molecules is hundreds of times greater than their size. Molecules generally move progressively and uniformly. After collisions, they begin to rotate.
AT liquids the distance between molecules is much smaller. Molecules vibrate and forward movement. Molecules at short intervals jump into new equilibrium positions (we observe the fluidity of a liquid).
AT solid Molecules in bodies oscillate and very rarely move (only with increasing temperature).
The third position of the molecular kinetic theory
There are interaction forces between molecules that are electromagnetic in nature. These forces make it possible to explain the emergence of elastic forces. When a substance is compressed, the molecules approach each other, a repulsive force arises between them, when external forces move the molecules away from each other (stretch the substance), between them there is a force of attraction.
Matter density
This is a scalar value, which is determined by the formula


Density of substances - known tabular values
Chemical characteristics of the substance
Avogadro constant N A- number of atoms contained in 12 g of carbon isotope
This video lesson is devoted to the topic “Basic provisions of the MKT. The structure of matter. Molecule". Here you will learn what molecular kinetic theory (MKT) studies in physics. Get acquainted with the three main provisions on which the ICT is based. Find out what defines physical properties substances and what an atom and a molecule are.
To begin with, let's recall all the previous sections of physics that we studied, and understand that all this time we have been considering processes that occur with macroscopic bodies (or objects of the macrocosm). Now we will study their structure and the processes occurring inside them.
Definition. macroscopic body- a body made up of a large number particles. For example: a car, a person, a planet, a billiard ball…
Microscopic body - a body made up of one or more particles. For example: atom, molecule, electron… (Fig. 1)

Rice. 1. Examples of micro and macro objects, respectively
Having thus determined the subject of study of the ICT course, we should now talk about the main goals that the ICT course sets for itself, namely:
- The study of processes occurring inside a macroscopic body (motion and interaction of particles)
- Properties of bodies (density, mass, pressure (for gases)…)
- The study of thermal phenomena (heating-cooling, changes in the state of aggregation of the body)
The study of these issues, which will take place throughout the entire topic, will now begin with the fact that we will formulate the so-called basic provisions of the ICT, that is, some statements, the truth of which has long been beyond doubt, and, starting from which, the entire further course will be built. .
Let's take them in turn:
All substances are made up of a large number of particles - molecules and atoms.
Definition. Atom- the smallest particle of a chemical element. The dimensions of atoms (their diameter) are of the order of cm. It is worth noting that there are relatively few different types of atoms, unlike molecules. All their varieties that are currently known to man are collected in the so-called periodic table (see Fig. 2)

Rice. 2. Periodic table chemical elements(essentially varieties of atoms) D. I. Mendeleev
Molecule- the structural unit of matter, consisting of atoms. Unlike atoms, they are larger and heavier than the latter, and most importantly, they have a huge variety.
A substance whose molecules are made up of one atom is called atomic, from more - molecular. For example: oxygen, water, salt () - molecular; helium silver (He, Ag) - atomic.
Moreover, it should be understood that the properties of macroscopic bodies will depend not only on quantitative characteristics their microscopic composition, but also from the quality.

If in the structure of atoms the substance has some definite geometry ( crystal lattice), or, conversely, does not have, then these bodies will have different properties. For example, amorphous bodies do not have a strict melting point. The best-known examples are amorphous graphite and crystalline diamond. Both substances are made up of carbon atoms.


Rice. 3. Graphite and diamond respectively
Thus, "out of how many, in which relative position and what atoms and molecules does matter consist of? - the first question, the answer to which will bring us closer to understanding the properties of bodies.
All the particles mentioned above are in continuous thermal chaotic motion.
Just as in the examples discussed above, it is important to understand not only the quantitative aspects of this movement, but also the qualitative ones for various substances.
Molecules and atoms of solids make only small vibrations relative to their permanent position; liquid - also oscillate, but due to the large size of the intermolecular space, they sometimes change places with each other; gas particles, in turn, practically without colliding, move freely in space.
Particles interact with each other.
This interaction is electromagnetic in nature (interactions of nuclei and electrons of an atom) and acts in both directions (both attraction and repulsion).

Here: d- distance between particles; a- particle size (diameter).
For the first time the concept of "atom" was introduced by the ancient Greek philosopher and naturalist Democritus (Fig. 4). In a later period, the Russian scientist Lomonosov actively asked himself the question of the structure of the microworld (Fig. 5).

Rice. 4. Democritus

Rice. 5. Lomonosov
In the next lesson, we will introduce methods of qualitative substantiation of the main provisions of the ICB.
Bibliography
- Myakishev G.Ya., Sinyakov A.Z. Molecular physics. Thermodynamics. - M.: Bustard, 2010.
- Gendenstein L.E., Dick Yu.I. Physics grade 10. - M.: Ileksa, 2005.
- Kasyanov V.A. Physics grade 10. - M.: Bustard, 2010.
- Elementy.ru ().
- Samlib.ru ().
- youtube().
Homework
- *Thanks to what force is it possible to make an experiment to measure the size of an oil molecule shown in the video tutorial?
- Why does the molecular kinetic theory not consider organic compounds?
- Why is even a very small grain of sand an object of the macrocosm?
- Forces of what nature predominantly act on particles from other particles?
- How to determine if a certain chemical structure a chemical element?
Basic provisions of molecular-kinetic theory.
Molecular-kinetic theory (MKT) deals with the study of the properties of substances, based on the ideas about the particles of matter.
The ICT is based on three main principles:
1. All substances are composed of particles - molecules, atoms and ions.
2. Particles of matter are constantly and randomly moving.
3. Particles of matter interact with each other.
Random (chaotic) movement of atoms and molecules in a substance is called thermal movement, because the speed of movement of particles increases with increasing temperature. Experimental confirmation of the continuous motion of atoms and molecules in matter is Brownian motion and diffusion.
Particles of matter.
All substances and bodies in nature consist of atoms and molecules - groups of atoms. Such large bodies are called macroscopic. Atoms and molecules are microscopic bodies. Modern instruments (ion projectors, tunneling microscopes) make it possible to see images of individual atoms and molecules.
The basis of the structure of matter is atoms. Atoms also have complex structure, they consist of elementary particles - protons, neutrons, which are part of the nucleus of an atom, electrons, as well as other elementary particles.
Atoms can combine into molecules, and there can be substances consisting only of atoms. Atoms as a whole are electrically neutral. Atoms that have too many or too few electrons are called ions. There are positive and negative ions.
Forces of interaction between molecules.
At very small distances between molecules, repulsive forces act. Due to this, molecules do not penetrate into each other and pieces of matter never shrink to the size of one molecule. A molecule is a complex system consisting of individual charged particles: electrons and atomic nuclei. Although, in general, the molecules are electrically neutral, significant electrical forces act between them at short distances: there is an interaction of electrons and atomic nuclei of neighboring molecules. If the molecules are located at distances exceeding their sizes by several times, then the interaction forces practically do not affect. Forces between electrically neutral molecules are short-range. At distances exceeding 2–3 molecular diameters, attractive forces act. As the distance between the molecules decreases, the force of attraction first increases, and then begins to decrease and decreases to zero, when the distance between two molecules becomes equal to the sum of the radii of the molecules. With a further decrease in the distance, the electron shells of the atoms begin to overlap, and rapidly increasing repulsive forces arise between the molecules.
Ideal gas. Basic equation of the MKT.
It is known that particles in gases, unlike liquids and solids, are located relative to each other at distances significantly exceeding their own dimensions. In this case, the interaction between molecules is negligible and the kinetic energy of molecules is much greater than the energy of intermolecular interaction. To find out the most common properties, inherent in all gases, use a simplified model real gases is an ideal gas. The main differences between an ideal gas and a real gas are:
1. Particles of an ideal gas are spherical bodies of very small sizes, practically material points.
2. There are no forces of intermolecular interaction between the particles.
3. Collisions of particles are absolutely elastic.
Real rarefied gases do indeed behave like an ideal gas. Let us use the ideal gas model to explain the origin of gas pressure. Due to thermal motion, gas particles from time to time hit the walls of the vessel. With each impact, the molecules act on the vessel wall with some force. Adding up with each other, the impact forces of individual particles form a certain pressure force that constantly acts on the wall. It is clear that the more particles are contained in the vessel, the more often they will hit the vessel wall, and the greater will be the pressure force, and hence the pressure. The faster the particles move, the harder they hit the vessel wall. Let's mentally imagine the simplest experience: A rolling ball hits a wall. If the ball rolls slowly, then it will hit the wall with less force on impact than if it were moving fast. The larger the particle mass, the greater the impact force. The faster the particles move, the more often they hit the walls of the vessel. So, the force with which the molecules act on the wall of the vessel is directly proportional to the number of molecules contained in a unit volume (this number is called the concentration of molecules and is denoted by n), the mass of the molecule m o , the mean square of their velocities and the area of the vessel wall. As a result, we obtain: the gas pressure is directly proportional to the concentration of particles, the mass of the particle, and the square of the particle's velocity (or their kinetic energy). The dependence of the pressure of an ideal gas on the concentration and on the average kinetic energy of the particles is expressed by the basic equation of the molecular-kinetic theory of an ideal gas. We have obtained the basic MKT equation for an ideal gas from general considerations, but it can be rigorously derived based on the laws of classical mechanics. Here is one of the forms of writing the main equation of the MKT:
P=(1/3) n m o V 2 .
Main results.
Molecular kinetic theory describes the behavior and properties of a special ideal object called ideal gas. This physical model is based on the molecular structure of matter. The creation of molecular theory is associated with the works of R. Clausius, J. Maxwell, D. Joule and L. Boltzmann.
Ideal gas. Molecular-kinetic theory of ideal gas is built on the following assumptions:
atoms and molecules can be considered as material points in continuous motion;
the intrinsic volume of gas molecules is negligible compared to the volume of the vessel;
all atoms and molecules are distinguishable, that is, it is possible in principle to follow the movement of each particle;
before the collision of gas molecules between them, there are no interaction forces, and the collisions of molecules between themselves and with the walls of the vessel are assumed to be absolutely elastic;
the motion of each atom or molecule of a gas is described by the laws of classical mechanics.
The laws obtained for an ideal gas can be used in the study of real gases. For this, experimental models of an ideal gas are created, in which the properties of a real gas are close to those of an ideal gas (for example, at low pressures and high temperatures).
Ideal gas laws
Boyle-Mariotte law:
for a given mass of gas at a constant temperature, the product of the gas pressure and its volume is a constant value: pV = const , (1.1)
at T = const , m = const .
Curve showing the relationship between quantities R and V, characterizes the properties of a substance at a constant temperature, and is called isotherm this is a hyperbola (Fig. 1.1.), and the process proceeding at a constant temperature is called isothermal.
Gay-Lussac's laws:
The volume of a given mass of gas at constant pressure varies linearly with temperature
V = V 0 (1 + t ) at P = const , m = const . (1.2)

p = p 0 (1 + t ) at V = const , m = const . (1.3)
In equations (1.2) and (1.3), temperature is expressed on the Celsius scale, pressure and volume - at
0 С, while
 .
.
A process that takes place at constant pressure is called isobaric, it can be represented as a linear function (Fig. 1.2.).
A process that takes place at constant volume is called isochoric(Fig. 1.3.).
It follows from equations (1.2) and (1.3) that isobars and isochores intersect the temperature axis at the point t =1/ \u003d - 273.15 С . If we move the origin to this point, then we move on to the Kelvin scale.
Introducing into formulas (1.2) and (1.3) thermodynamic temperature, the laws of Gay-Lussac can be given a more convenient form:
V = V 0 (1+t) = = V 0 = =V 0 T;
p = p 0 (1+t) = p 0 = p 0 T;
 at
p=const, m=const
;
(1.4)
at
p=const, m=const
;
(1.4)
 at V = const, m = const
,
(1.5)
at V = const, m = const
,
(1.5)
where indices 1 and 2 refer to arbitrary states lying on the same isobar or isochore .
Avogadro's Law:
moles of any gases at the same temperatures and pressures occupy the same volumes.
Under normal conditions, this volume is equal to V,0 \u003d 22.4110 -3 m 3 / mol . By definition, one mole of different substances contains the same number of molecules, equal to constant Avogadro:N A = 6,02210 23 mol -1 .
Dalton's law:
the pressure of a mixture of different ideal gases is equal to the sum of the partial pressures R 1 , R 2 , R 3 … R n, gases included in it:
p = p 1 + p 2 + R 3 + …+ p n .
Partial pressure – this is the pressure that a gas in a gas mixture would produce if it alone occupied a volume equal to the volume of the mixture at the same temperature.
Ideal gas equation of state
(Clapeyron-Mendeleev equation)
There is a definite relationship between temperature, volume and pressure. This relationship can be represented by a functional dependency:
f(p, V, T)= 0.
In turn, each of the variables ( p, v, t) is a function of two other variables. The type of functional dependence for each phase state of a substance (solid, liquid, gaseous) is found experimentally. This is a very laborious process and the equation of state has been established only for gases that are in a rarefied state, and in an approximate form for some compressed gases. For substances that are not in a gaseous state, this problem has not yet been solved.
The French physicist B. Clapeyron brought ideal gas equation of state, by combining the laws of Boyle-Mariotte, Gay-Lussac, Charles:
 . (1.6)
. (1.6)
Expression (1.6) is the Clapeyron equation, where AT is the gas constant. It is different for different gases.
DI. Mendeleev combined Clapeyron's equation with Avogadro's law, referring equation (1.6) to one mole and using the molar volume V . According to Avogadro's law, for the same R and T moles of all gases occupy the same molar volume V .
.
Therefore, the constant AT will be the same for all ideal gases. This constant is usually denoted R and equal to R=
8,31
 .
.
Clapeyron-Mendeleev equation has the following form:
p V . = R T.
From equation (1.7) for one mole of gas, one can go to to the Clapeyron-Mendeleev equation for an arbitrary mass of gas:
 , (1.7)
, (1.7)
where
–
molar mass
(mass of one mole of substance, kg/mol); m
mass of gas;
 - amount of matter .
- amount of matter .
More often, another form of the ideal gas equation of state is used, introducing Boltzmann's constant:  .
.
Then equation (1.7) looks like this:
 ,
(1.8)
,
(1.8)
where
 –
concentration of molecules (number of molecules per unit volume). It follows from this expression that the pressure of an ideal gas is directly proportional to the concentration of its molecules or the density of the gas. At the same temperatures and pressures, all gases contain the same number of molecules per unit volume. The number of molecules contained in 1 m 3 under normal conditions is called
Loschmidt number:
–
concentration of molecules (number of molecules per unit volume). It follows from this expression that the pressure of an ideal gas is directly proportional to the concentration of its molecules or the density of the gas. At the same temperatures and pressures, all gases contain the same number of molecules per unit volume. The number of molecules contained in 1 m 3 under normal conditions is called
Loschmidt number:
N L = 2.68 10 25 m -3.
Basic equation of molecular kinetic
theory of ideal gases
The most important task The kinetic theory of gases is the theoretical calculation of the pressure of an ideal gas based on molecular kinetic concepts. The basic equation of the molecular kinetic theory of ideal gases is derived using statistical methods.
It is assumed that the gas molecules move randomly, the number of mutual collisions between the gas molecules is negligible compared to the number of impacts on the walls of the vessel, and these collisions are absolutely elastic. On the wall of the vessel, some elementary area S and calculate the pressure that the gas molecules will exert on this area.
It is necessary to take into account the fact that the molecules can actually move towards the site at different angles and can have different velocities, which, moreover, can change with each collision. In theoretical calculations, the chaotic motions of molecules are idealized, they are replaced by motion along three mutually perpendicular directions.
If we consider a vessel in the form of a cube, in which N gas molecules in six directions, it is easy to see that at any moment 1/3 of the number of all molecules moves along each of them, and half of them (that is, 1/6 of the number of all molecules) moves in one direction, and the second half (also 1/6) - in the opposite direction. With each collision, an individual molecule moving perpendicular to the site, reflecting, transfers momentum to it, while its momentum (momentum) changes by the amount
R 1 =m 0 v – (– m 0 v) = 2 m 0 v.
The number of impacts of molecules moving in a given direction on the site will be equal to: N = 1/6 n Svt. When colliding with the platform, these molecules will transfer momentum to it.
P= N P 1 =2 m 0 vnSvt= m 0 v 2 nSt,
where n is the concentration of molecules. Then the pressure that the gas exerts on the wall of the vessel will be equal to:
p =  =
n m 0
v 2
.
(1.9)
=
n m 0
v 2
.
(1.9)
However, gas molecules move at different speeds: v 1 , v 2 , …,v n, so the velocities must be averaged. The sum of the squares of the velocities of the gas molecules, divided by their number, determines the root mean square velocity:
 .
.
Equation (1.9) will take the form:
 (1.10)
(1.10)
expression (1.10) is called the basic equation of molecular kinetic theory ideal gases.
Given that  , we get:
, we get:
p V = N  =E,
(1.11)
=E,
(1.11)
where E is the total kinetic energy of the translational motion of all gas molecules. Therefore, the gas pressure is directly proportional to the kinetic energy of the translational motion of the gas molecules.
For one mole of gas m =, and the Clapeyron-Mendeleev equation has the following form:
p V . = R T,
and since it follows from (1.11) that p V . = v sq. 2 , we get:
R.T.= v sq. 2 .
Hence, the root-mean-square velocity of gas molecules is equal to
v
sq.
=
 =
= =
= ,
,
where k = R/N A = 1.3810 -23 J/K – Boltzmann's constant. From here you can find the mean square velocity of oxygen molecules at room temperature - 480 m/s, hydrogen - 1900 m/s.
Molecular-kinetic meaning of temperature
Temperature is a quantitative measure of how hot a body is. To clarify the physical meaning of the absolute thermodynamic temperature T Let's compare the basic equation of the molecular-kinetic theory of gases (1.14) with the Clapeyron-Mendeleev equation p V = R.T.
Equating the right parts of these equations, we find the average value of the kinetic energy 0 of one molecule ( = N/N A , k=R/N A):
 .
.
The most important conclusion of the molecular kinetic theory follows from this equation: the average kinetic energy of the translational motion of one molecule of an ideal gas depends only on temperature, while it is directly proportional to thermodynamic temperature. Thus, the thermodynamic temperature scale acquires a direct physical meaning: at T= 0 the kinetic energy of ideal gas molecules is zero. Therefore, based on this theory, the translational motion of the gas molecules will stop and its pressure will become equal to zero.
Theory of equilibrium properties of an ideal gas
Number of degrees of freedom of molecules. The molecular-kinetic theory of ideal gases leads to a very important consequence: gas molecules move randomly, and the average kinetic energy of the translational motion of the molecule is determined solely by temperature.
The kinetic energy of molecular motion is not exhausted by the kinetic forward motion energy: it also consists of kinetic energies rotation and fluctuations molecules. In order to calculate the energy going into all types of molecular motion, it is necessary to define number of degrees of freedom.
Under number of degrees of freedom (i) of the body is implied the number of independent coordinates that must be entered to determine the position of the body in space.
H  For example, a material point has three degrees of freedom, since its position in space is determined by three coordinates: x, y and z. Therefore, a monatomic molecule has three degrees of freedom of translational motion.
For example, a material point has three degrees of freedom, since its position in space is determined by three coordinates: x, y and z. Therefore, a monatomic molecule has three degrees of freedom of translational motion.
D  a buchatomic molecule has 5 degrees of freedom (Fig. 1.4): 3 degrees of freedom of translational motion and 2 degrees of freedom of rotational motion.
a buchatomic molecule has 5 degrees of freedom (Fig. 1.4): 3 degrees of freedom of translational motion and 2 degrees of freedom of rotational motion.
Molecules of three or more atoms have 6 degrees of freedom: 3 degrees of freedom of translational motion and 3 degrees of freedom of rotational motion (Fig. 1.5).
Each gas molecule has a certain number of degrees of freedom, three of which correspond to its translational motion.
Regulation on the equal distribution of energy
by degrees of freedom
The basic premise of the molecular-kinetic theory of gases is the assumption of complete randomness in the motion of molecules. This applies to both oscillatory and rotational movements, and not just translational. It is assumed that all directions of motion of molecules in a gas are equally probable. Therefore, we can assume that for each degree of freedom of a molecule, on average, there is the same amount of energy - this is the position on the equipartition of energy over degrees of freedom. The energy per one degree of freedom of a molecule is:
 . (1.12)
. (1.12)
If the molecule has i degrees of freedom, then for each degree of freedom there is on average:
 .
(1.13)
.
(1.13)
Internal energy of an ideal gas
If we attribute the total supply of internal energy of the gas to one mole, then we obtain its value by multiplying by the Avogadro number:
 .
(1.14)
.
(1.14)
Hence it follows that internal energy one mole of an ideal gas depends only on the temperature and the number of degrees of freedom of the gas molecules.
Maxwell and Boltzmann distributions
Distribution of molecules of an ideal gas in terms of velocities and energies of thermal motion (Maxwell distribution). At a constant gas temperature, all directions of molecular motion are assumed to be equally probable. In this case, the root-mean-square velocity of each molecule remains constant and is equal to
 .
.
This is explained by the fact that in an ideal gas, which is in a state of equilibrium, a certain stationary velocity distribution of molecules that does not change with time is established. this distribution is subject to a certain statistical law, which was theoretically derived by J. Maxwell. Maxwell's law is described by the function
 ,
,
that is the function f(v) determines the relative number of molecules  , whose velocities lie in the interval from v
before v+dv. Applying the methods of probability theory, Maxwell found the law of distribution of molecules of an ideal gas in terms of velocities:
, whose velocities lie in the interval from v
before v+dv. Applying the methods of probability theory, Maxwell found the law of distribution of molecules of an ideal gas in terms of velocities:
 . (1.15)
. (1.15)
The distribution function is shown graphically in fig. 1.6. The area bounded by the distribution curve and the x-axis is equal to one. This means that the function f(v) satisfies the normalization condition:
 .
.
FROM  velocity at which the distribution function of ideal gas molecules in terms of velocities f(v) is maximum, is called most likely
speed
v B .
velocity at which the distribution function of ideal gas molecules in terms of velocities f(v) is maximum, is called most likely
speed
v B .
Values v = 0 and v = correspond to the minima of expression (1.15). The most probable speed can be found by differentiating expression (1.23) and equating it to zero:
 =
= =
1,41
=
1,41
With an increase in temperature, the maximum of the function will shift to the right (Fig. 1.6), that is, with an increase in temperature, the most probable speed also increases, however, the area bounded by the curve remains unchanged. It should be noted that in gases and at low temperatures there is always a small number of molecules that move at high speeds. The presence of such "hot" molecules has great importance during many processes.
Arithmetic average speed molecules is determined by the formula
 .
.
Root mean square speed
 =
1,73
=
1,73 .
.
The ratio of these velocities does not depend on temperature or on the type of gas.
Distribution function of molecules by thermal motion energies. This function can be obtained by substituting the value of kinetic energy instead of velocity into the distribution equation of molecules (1.15):
 .
.
Having integrated the expression over the energy values from  before
before 
 , we get average kinetic energy ideal gas molecules:
, we get average kinetic energy ideal gas molecules:
 .
.
barometric formula. Boltzmann distribution. When deriving the basic equation of the molecular-kinetic theory of gases and the Maxwell distribution of molecules by velocities, it was assumed that the molecules of an ideal gas are not affected by external forces, therefore the molecules are uniformly distributed throughout the volume. However, the molecules of any gas are in the Earth's gravitational field. When deriving the law of dependence of pressure on height, it is assumed that the gravitational field is uniform, the temperature is constant and the mass of all molecules is the same:
 . (1.16)
. (1.16)
Expression (1.16) is called barometric formula. It allows you to find the atmospheric pressure depending on the altitude, or by measuring the pressure, you can find the altitude. Because h 1 is the height above sea level, where the pressure is considered normal, then the expression can be modified:
 .
.
The barometric formula can be converted using the expression p = nkT:
 ,
,
G  de n
–
concentration of molecules at altitude h,
m 0
gh=P–
potential energy of a molecule in a gravitational field. At constant temperature, the density of the gas is greater where the potential energy of the molecule is lower. Graphically, the law of decrease in the number of particles per unit volume with height looks as shown in Fig. 1.7.
de n
–
concentration of molecules at altitude h,
m 0
gh=P–
potential energy of a molecule in a gravitational field. At constant temperature, the density of the gas is greater where the potential energy of the molecule is lower. Graphically, the law of decrease in the number of particles per unit volume with height looks as shown in Fig. 1.7.
For an arbitrary external potential field, we write the following general expression
 ,
,